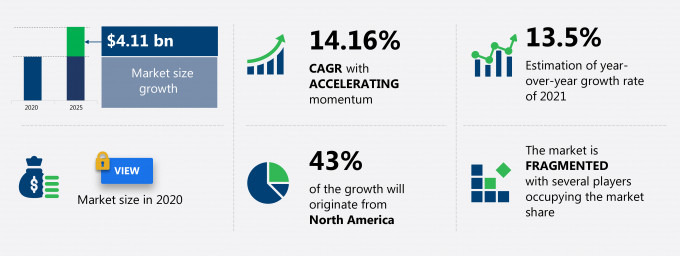
The autologous cell therapy market share is expected to increase by USD 4.11 billion from 2020 to 2025, and the market’s growth momentum will accelerate at a CAGR of 14.16%.
This autologous cell therapy market research report provides valuable insights on the post COVID-19 impact on the market, which will help companies evaluate their business approaches. Furthermore, this report extensively covers autologous cell therapy market segmentation by product (autologous stem cell therapy and autologous cellular immunotherapies) and geography (North America, Europe, APAC, MEA, and South America). The autologous cell therapy market report also offers information on several market vendors, including Bayer AG, Brainstorm Cell Therapeutics Inc., Daiichi Sankyo Co. Ltd., FUJIFILM Holdings Corp., Holostem Terapie Avanzate Srl, Osiris Therapeutics Inc., Takeda Pharmaceutical Co. Ltd., Teva Pharmaceutical Industries Ltd., Sumitomo Chemical Co. Ltd., and Vericel Corp. among others.
What will the Autologous Cell Therapy Market Size be During the Forecast Period?
Download the Free Report Sample to Unlock the Autologous Cell Therapy Market Size for the Forecast Period and Other Important Statistics
Autologous Cell Therapy Market: Key Drivers, Trends, and Challenges
The increasing demand for effective drugs for cardiac and degenerative disorders is notably driving the autologous cell therapy market growth, although factors such as critical ethical challenges with respect to stem cell research may impede the market growth. Our research analysts have studied the historical data and deduced the key market drivers and the COVID-19 pandemic impact on the autologous cell therapy industry. The holistic analysis of the drivers will help in deducing end goals and refining marketing strategies to gain a competitive edge.
Key Autologous Cell Therapy Market Driver
The increasing demand for effective drugs for cardiac and degenerative disorders is a major factor driving the global autologous cell therapy market share growth. There has been an increased demand for providing effective drugs for cardiac and degenerative disorders globally. Prior to the advent of autologous cell therapies, there was no effective drug to repair a damaged heart. The discovery of possible cardiac autologous cells opened new possibilities for repairing damaged cardiac tissue caused by acute myocardial infarction or coronary artery disease. There are approximately 19 product candidates being developed for the treatment of cardiac disorders, with eight in Phase III and six in Phase II development stages. Some of the companies that are developing these drugs include Shire, Athersys, Japan Regenerative Medicine, MyoCell, and Celyad. Mesoblast is developing MPC-150-IM, a Phase III candidate for the treatment of advanced and end-stage chronic heart failure. Also, in July 2019, Bayer AG provided about $250 million to Century Therapeutics for the development of next-generation immune-oncology therapeutics. In addition, Shire has been developing autologous stem cell therapies for chronic myocardial ischemia. These products are expected to be launched during the forecast period and will have a substantial impact on market growth.
Key Autologous Cell Therapy Market Trend
The increasing M&A activities is another factor supporting the global autologous cell therapy market share growth. M&A activities have the potential to enable vendors to explore additional opportunities without incurring a huge financial burden. Such alliances for product development and product commercialization provide vendors with multiple benefits, such as cost containment, extended product lines, and increased geographical reach. This is expected to support the growth of the market during the forecast period.
Key Autologous Cell Therapy Market Challenge
The critical ethical challenges with respect to stem cell research will be a major challenge for the global autologous cell therapy market share growth during the forecast period. Over the years, there has been a continuous debate over ethical issues related to stem cell therapy. Embryonic stem cell development is continuously debated by scientists and policymakers. The use of human embryonic stem cells in research has always concerned decision-makers and the scientific fraternity. Some of the issues include the cloning of embryonic stem cells and the destruction of embryos to create cell lines. Moreover, concerns regarding safety, scientific purity, and consent to the use of human embryonic stem cell lines for research purposes have always been points of concern. Also, stem cell therapy requires experiments and clinical trials on animals. With growing ethical concerns globally, animal testing must be regulated by government agencies to minimize the use of animals in research. Therefore, the presence of ethical issues may act as a hindrance to the ongoing research in stem cell therapy and may inhibit market growth during the forecast period.
This autologous cell therapy market analysis report also provides detailed information on other upcoming trends and challenges that will have a far-reaching effect on the market growth. The actionable insights on the trends and challenges will help companies evaluate and develop growth strategies for 2021-2025.
Parent Market Analysis
Technavio categorizes the global autologous cell therapy market as part of the global pharmaceutical market. Our research report has extensively covered external factors influencing the parent market growth potential in the coming years, which will determine the levels of growth of the autologous cell therapy market during the forecast period.
Who are the Major Autologous Cell Therapy Market Vendors?
The report analyzes the market’s competitive landscape and offers information on several market vendors, including:
- Bayer AG
- Brainstorm Cell Therapeutics Inc.
- Daiichi Sankyo Co. Ltd.
- FUJIFILM Holdings Corp.
- Holostem Terapie Avanzate Srl
- Osiris Therapeutics Inc.
- Takeda Pharmaceutical Co. Ltd.
- Teva Pharmaceutical Industries Ltd.
- Sumitomo Chemical Co. Ltd.
- Vericel Corp.
This statistical study of the autologous cell therapy market encompasses successful business strategies deployed by the key vendors. The autologous cell therapy market is fragmented and the vendors are deploying organic and inorganic growth strategies to compete in the market.
Product Insights and News
- Bayer AG- The company offers induced pluripotent stem cells, which are developed by reprogramming mature body cells to behave like embryonic stem cells that are injected to restore diseased tissue in patients.
To make the most of the opportunities and recover from post-COVID-19 impact, market vendors should focus more on the growth prospects in the fast-growing segments, while maintaining their positions in the slow-growing segments.
The autologous cell therapy market forecast report offers in-depth insights into key vendor profiles. The profiles include information on the production, sustainability, and prospects of the leading companies.
Autologous Cell Therapy Market Value Chain Analysis
Our report provides extensive information on the value chain analysis for the autologous cell therapy market, which vendors can leverage to gain a competitive advantage during the forecast period. The end-to-end understanding of the value chain is essential in profit margin optimization and evaluation of business strategies. The data available in our value chain analysis segment can help vendors drive costs and enhance customer services during the forecast period.
The value chain of the global pharmaceutical market includes the following core components:
- Inputs
- Inbound logistics
- Operations
- Outbound logistics
- Marketing and sales
- Service
- Support activities
- Innovation
The report has further elucidated other innovative approaches being followed by manufacturers to ensure a sustainable market presence.
Which are the Key Regions for Autologous Cell Therapy Market?
For more insights on the market share of various regions Request for a FREE sample now!
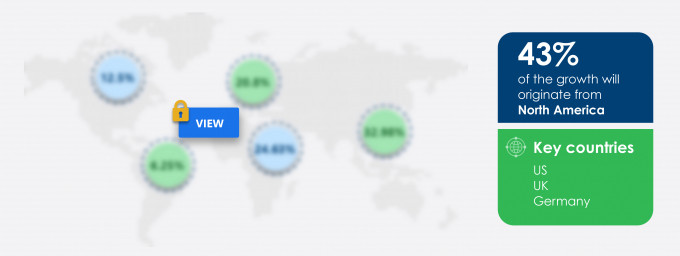
43% of the market’s growth will originate from North America during the forecast period. US and Canada are the key markets for the autologous cell therapy market in North America. Market growth in this region will be slower than the growth of the market in APAC.
The limitations in traditional organ transplantations fueling demand for stem cell therapies will facilitate the autologous cell therapy market growth in North America over the forecast period. This market research report entails detailed information on the competitive intelligence, marketing gaps, and regional opportunities in store for vendors, which will assist in creating efficient business plans.
What are the Revenue-generating Product Segments in the Autologous Cell Therapy Market?
To gain further insights on the market contribution of various segments Request for a FREE sample
The autologous cell therapy market share growth in the autologous stem cell therapy segment will be significant during the forecast period. The autologous stem cell therapy segment is growing at a medium pace due to its manufacturing complexities. In addition, the clinical evidence is less established in the autologous stem cell therapy segment. Also, this therapy is less preferred in emergency care as it requires more time to produce autologous therapy cells. However, fewer complications associated with the use of autologous stem cell therapy have driven vendors to conduct extensive clinical trials.
This report provides an accurate prediction of the contribution of all the segments to the growth of the autologous cell therapy market size and actionable market insights on post COVID-19 impact on each segment.
|
Autologous Cell Therapy Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
120 |
|
Base year |
2020 |
|
Forecast period |
2021-2025 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 14.16% |
|
Market growth 2021-2025 |
$ 4.11 billion |
|
Market structure |
Fragmented |
|
YoY growth (%) |
13.5 |
|
Regional analysis |
North America, Europe, APAC, MEA and South America |
|
Performing market contribution |
North America at 43% |
|
Key consumer countries |
US, UK, Germany, Canada, and Japan |
|
Competitive landscape |
Leading companies, Competitive strategies, Consumer engagement scope |
|
Key companies profiled |
Bayer AG, Brainstorm Cell Therapeutics Inc., Daiichi Sankyo Co. Ltd., FUJIFILM Holdings Corp., Holostem Terapie Avanzate Srl, Osiris Therapeutics Inc., Takeda Pharmaceutical Co. Ltd., Teva Pharmaceutical Industries Ltd., Sumitomo Chemical Co. Ltd., and Vericel Corp. |
|
Market dynamics |
Parent market analysis, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, COVID 19 impact and recovery analysis and future consumer dynamics, Market condition analysis for forecast period |
|
Customization purview |
If our report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this Autologous Cell Therapy Market Report?
- CAGR of the market during the forecast period 2021-2025
- Detailed information on factors that will drive autologous cell therapy market growth during the next five years
- Precise estimation of the autologous cell therapy market size and its contribution to the parent market
- Accurate predictions on upcoming trends and changes in consumer behavior
- The growth of the autologous cell therapy industry across North America, Europe, APAC, MEA, and South America
- A thorough analysis of the market’s competitive landscape and detailed information on vendors
- Comprehensive details of factors that will challenge the growth of autologous cell therapy market vendors
We can help! Our analysts can customize this report to meet your requirements. Get in touch