Preclinical CRO Market Size 2025-2029
The preclinical cro market size is valued to increase USD 2.73 billion, at a CAGR of 8.1% from 2024 to 2029. Increasing prevalence of chronic diseases will drive the preclinical cro market.
Major Market Trends & Insights
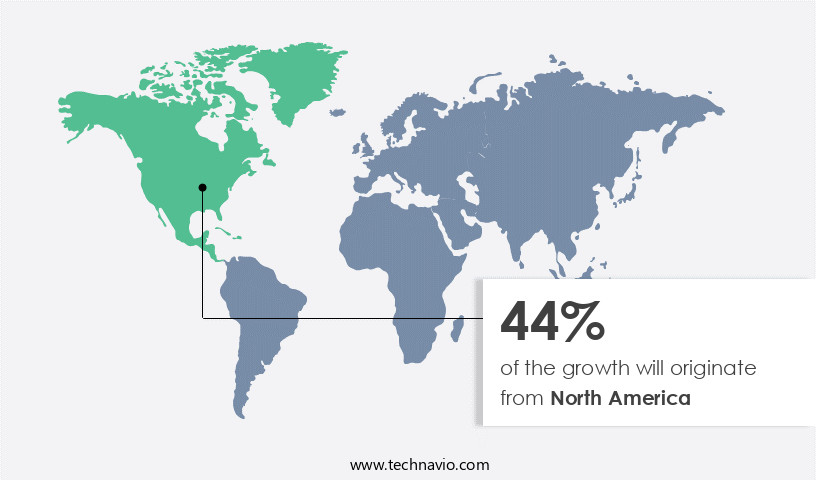
- North America dominated the market and accounted for a 44% growth during the forecast period.
- By End-user - P and B companies segment was valued at USD 2.74 billion in 2023
- By Service - Toxicology testing segment accounted for the largest market revenue share in 2023
Market Size & Forecast
- Market Opportunities: USD 97.62 million
- Market Future Opportunities: USD 2734.80 million
- CAGR from 2024 to 2029 : 8.1%
Market Summary
- The Preclinical Contract Research Organizations (CRO) Market represents a dynamic and continually evolving landscape, driven by the increasing prevalence of chronic diseases and the growing number of clinical trials. With the global burden of chronic diseases on the rise, the demand for efficient and effective preclinical research services has surged. According to recent reports, the market is expected to account for over 40% of the overall CRO market share. Core technologies and applications, such as high-throughput screening, genomics, and proteomics, are revolutionizing preclinical research, enabling faster and more accurate drug discovery. Service types, including toxicology, bioanalysis, and preclinical development, are in high demand as pharmaceutical and biotech companies seek to bring new therapies to market.
- Despite these opportunities, the market faces challenges, including intellectual property issues and regulatory compliance. Intellectual property disputes can hinder innovation and collaboration, while stringent regulations, such as the European Medicines Agency's (EMA) and the U.S. Food and Drug Administration's (FDA) guidelines, require CROs to maintain the highest standards of quality and data integrity. In summary, the market is a vibrant and evolving industry, characterized by the increasing prevalence of chronic diseases, the growing number of clinical trials, and the ongoing development of advanced technologies and applications. Despite challenges, such as intellectual property issues and regulatory compliance, the market offers significant opportunities for growth and innovation.
What will be the Size of the Preclinical CRO Market during the forecast period?
Get Key Insights on Market Forecast (PDF) Request Free Sample
How is the Preclinical CRO Market Segmented ?
The preclinical cro industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- End-user
- P and B companies
- Medical device companies
- Academic institutes
- Service
- Toxicology testing
- Bioanalysis and DMPK studies
- Compound management
- Others
- Geography
- North America
- US
- Canada
- Mexico
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- Rest of World (ROW)
- North America
By End-user Insights
The p and b companies segment is estimated to witness significant growth during the forecast period.
Pharmaceutical and biopharmaceutical companies increasingly turn to Contract Research Organizations (CROs) to expand their capabilities without adding unnecessary overhead. Over the past two decades, CROs have grown in significance within the industry due to several factors. The rising demand for generic drugs and biologics, the capital-intensive nature of pharmaceutical manufacturing, and the complex requirements for safety assessments and regulatory submissions have made CROs indispensable partners. In 2023, several notable trends emerged. For instance, there was a surge in demand for non-clinical studies, including pharmacokinetic studies, genotoxicity, carcinogenicity, and safety pharmacology. CROs also played a crucial role in study conduct, data management, statistical analysis, and quality assurance for in vitro and in vivo studies.
Furthermore, CROs provided essential services such as formulation development, lead optimization, target identification, bioanalytical services, and ADME profiling. With GLP compliance and good laboratory practices, CROs ensured the highest standards for data interpretation, regulatory submissions, and biomarker discovery during preclinical development. These enabling studies are vital for clinical trial design and eventual market approval.
The P and B companies segment was valued at USD 2.74 billion in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
North America is estimated to contribute 44% to the growth of the global market during the forecast period.Technavio’s analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
See How Preclinical CRO Market Demand is Rising in North America Request Free Sample
In the global preclinical Contract Research Organization (CRO) market, North America holds a significant position, accounting for a substantial share. Major players in this region include IQVIA, Laboratory Corporation of America, and Parexel, all headquartered in the US. These companies offer contract research solutions and innovative technology services to a multitude of large pharmaceutical companies and biotechnology firms. The dependence on these CROs is mutual, as small- and medium-sized entities in the region also benefit from their offerings.
The market's growth can be attributed to the increasing demand for outsourcing research activities to specialized organizations, aiming to reduce costs and enhance efficiency. Companies in the region cater to a global clientele, further emphasizing the market's robustness.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
The preclinical Contract Research Organization (CRO) market plays a pivotal role in the global drug development landscape, offering a range of services from preclinical safety assessment studies to regulatory compliant data management for trials. These CROs specialize in non-clinical drug development services, including pharmacokinetic and pharmacodynamic analysis, in vivo and in vitro pharmacology studies, and biomarker discovery and validation for drug development. Preclinical safety assessment studies form the foundation of the drug development process, ensuring the safety of potential therapeutics before human trials. These studies encompass a range of services, such as GLP compliant toxicology testing, safety pharmacology testing for regulatory submission, and histopathology evaluation for toxicology studies.
Comprehensive bioanalytical testing services are also essential, providing detailed toxicology reports for regulatory submissions and drug metabolism and pharmacokinetics characterization. The market is highly competitive, with a significant number of players offering comprehensive preclinical drug development support. Animal model selection for preclinical studies is crucial, and CROs leverage their expertise in study design and execution to deliver accurate and reliable results. In vivo and in vitro efficacy testing is another critical service, enabling clients to assess the therapeutic potential of their drug candidates. Compared to traditional laboratory settings, the adoption of advanced technologies in preclinical CROs has significantly increased efficiency and accuracy.
For instance, the use of automated systems for high-throughput screening and data analysis has led to faster turnaround times and more precise results. Additionally, the integration of artificial intelligence and machine learning algorithms has streamlined the data analysis process, enabling CROs to deliver more actionable insights to their clients. Despite the growing competition, a few key players dominate the high-end the market, accounting for a substantial share. These players offer a broader range of services and have established a strong reputation for delivering high-quality data and reliable results. As the demand for innovative therapeutics continues to grow, the market is expected to expand, offering significant opportunities for both established players and new entrants.
What are the key market drivers leading to the rise in the adoption of Preclinical CRO Industry?
- The rising prevalence of chronic diseases serves as the primary market driver, significantly expanding the market scope.
- Chronic diseases, including cardiovascular diseases (CVDs), cancer, chronic obstructive pulmonary disease (COPD), and type-2 diabetes, pose significant health challenges worldwide. These conditions are frequently linked to risk factors like high blood pressure (BP), blood cholesterol, and obesity. For instance, approximately 60% of American adults were managing at least one chronic disease in 2023. Unhealthy diets, insufficient physical activity, and tobacco use contribute to the development of these diseases. Hypertension, a common chronic condition, is often referred to as a "silent killer" due to its lack of warning signs or symptoms.
- The prevalence of hypertension is particularly high in low- and middle-income countries. Dietary modifications, regular exercise, and tobacco cessation are essential preventative measures against chronic diseases. The ongoing global health crisis underscores the importance of prioritizing chronic disease management and prevention.
What are the market trends shaping the Preclinical CRO Industry?
- The increasing number of clinical trials is a notable market trend. Clinical trials are becoming more prevalent in the current market landscape.
- The clinical research sector is experiencing significant growth due to the increasing demand for new drugs, biologics, and medical devices to address chronic and epidemic diseases. Private entities, including drug manufacturers and biologics producers, are key contributors to clinical trials through funding and research support. To minimize research expenses and optimize clinical trial processes, outsourcing preclinical trials to Contract Research Organizations (CROs) is a prevalent trend in developed nations like the US, Canada, and the UK. Government initiatives also play a crucial role in fostering clinical trials' expansion.
- In various countries, governments fund drug manufacturers and provide essential training to clinical trial staff to facilitate the clinical trial process in regional markets or specific nations. This dynamic market landscape underscores the importance of effective collaboration between various stakeholders to advance clinical research and ultimately improve patient care.
What challenges does the Preclinical CRO Industry face during its growth?
- The CRO industry's growth is being impeded by intellectual property issues, posing a significant challenge that necessitates careful navigation to ensure compliance and foster industry expansion.
- The preclinical Contract Research Organizations (CRO) market plays a pivotal role in supporting the pharmaceutical, biotechnology, and medical device industries by offering outsourced research services. However, intellectual property concerns pose a significant challenge to this market's expansion. With the majority of medical devices and medication candidates being patented, outsourcing clinical trials to a CRO carries the risk of data leakage. Pharmaceutical companies, which develop innovative solutions, must submit clinical data on safety and efficacy to regulatory agencies for drug approval.
- These regulatory bodies stringently enforce data security measures to safeguard intellectual property. Consequently, CROs must invest in robust data security systems and adhere to regulatory guidelines to mitigate these concerns. This continuous focus on data security and regulatory compliance underscores the evolving nature of the market.
Exclusive Technavio Analysis on Customer Landscape
The preclinical cro market forecasting report includes the adoption lifecycle of the market, covering from the innovator’s stage to the laggard’s stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the preclinical cro market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape of Preclinical CRO Industry
Competitive Landscape
Companies are implementing various strategies, such as strategic alliances, preclinical cro market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Altasciences - This company specializes in preclinical contract research organization services, providing both GLP and non-GLP in vivo studies to support pharmaceutical and biotech industry research and development. Their expertise encompasses various therapeutic areas, ensuring rigorous scientific standards and regulatory compliance.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Altasciences
- AmplifyBio
- BioEmission Technology Solutions
- Charles River Laboratories International Inc.
- Crown Bioscience
- CYNBIOSE
- Eurofins Scientific SE
- Gempharmatech Co. Ltd.
- Global Center for Medical Innovation
- ICON plc
- Imavita S.A.S.
- IQVIA Holdings Inc.
- Kunming Biomed International Ltd.
- Laboratory Corp. of America Holdings
- Medpace Holdings Inc.
- Parexel International Corp.
- PPD
- Veeda Clinical Research Ltd.
- Vivotecnia
- WuXi AppTec Co. Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Preclinical CRO Market
- In January 2024, Quintiles IMS (now IQVIA) announced the launch of its new preclinical services platform, "IQVIA Preclinical," aimed at streamlining the drug discovery process for biotech and pharmaceutical companies (IQVIA press release). In March 2024, Charles River Laboratories International, Inc. Entered into a strategic partnership with Merck KGaA to expand their collaboration on early-stage research and development projects (Merck KGaA press release).
- In April 2024, Covance, a Labcorp company, secured a significant investment of USD250 million to expand its preclinical research capabilities and strengthen its position in the market (Labcorp press release). In May 2025, PPD Inc. Received FDA approval for its new preclinical research facility in Singapore, enabling the company to offer expanded services to clients in the Asia-Pacific region (PPD press release). These developments underscore the growing demand for comprehensive preclinical services and the ongoing competition among leading CROs to expand their offerings and geographic reach.
Dive into Technavio’s robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Preclinical CRO Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
190 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 8.1% |
|
Market growth 2025-2029 |
USD 2734.8 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
7.6 |
|
Key countries |
US, Germany, Canada, UK, China, France, India, Japan, Italy, and Mexico |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
Research Analyst Overview
- In the dynamic and intricate realm of preclinical research, various studies play a pivotal role in shaping the drug development landscape. These investigations span a broad spectrum, encompassing study protocols for pharmacokinetic assessments, statistical analysis of data, and genetic toxicology evaluations, all adhering to GLP compliance. Efficacy studies, carcinogenicity assessments, and safety pharmacology investigations are integral components of the preclinical development process. Quality assurance measures ensure the reliability and accuracy of data, while data management and interpretation facilitate informed decision-making. Regulatory submissions are a crucial step in the journey towards bringing new drug candidates to market.
- In vitro studies, such as those focusing on reproductive toxicology and biomarker discovery, provide valuable insights into drug effects. DMPK analysis, bioanalytical services, and contract research organizations contribute significantly to drug candidate selection and formulation development. Lead optimization and target identification are essential stages in the preclinical development pipeline, with in vivo studies and toxicology testing offering critical information on drug safety and efficacy. Non-clinical studies, including ADME profiling and safety assessments, provide essential data for clinical trial design. Ind enabling studies further refine the understanding of drug properties and potential interactions. Preclinical development is an ongoing process, with continuous advancements in technology and research methods shaping the market landscape.
What are the Key Data Covered in this Preclinical CRO Market Research and Growth Report?
-
What is the expected growth of the Preclinical CRO Market between 2025 and 2029?
-
USD 2.73 billion, at a CAGR of 8.1%
-
-
What segmentation does the market report cover?
-
The report is segmented by End-user (P and B companies, Medical device companies, and Academic institutes), Service (Toxicology testing, Bioanalysis and DMPK studies, Compound management, and Others), and Geography (North America, Europe, Asia, and Rest of World (ROW))
-
-
Which regions are analyzed in the report?
-
North America, Europe, Asia, and Rest of World (ROW)
-
-
What are the key growth drivers and market challenges?
-
Increasing prevalence of chronic diseases, Intellectual property issues hampering CRO industry growth
-
-
Who are the major players in the Preclinical CRO Market?
-
Altasciences, AmplifyBio, BioEmission Technology Solutions, Charles River Laboratories International Inc., Crown Bioscience, CYNBIOSE, Eurofins Scientific SE, Gempharmatech Co. Ltd., Global Center for Medical Innovation, ICON plc, Imavita S.A.S., IQVIA Holdings Inc., Kunming Biomed International Ltd., Laboratory Corp. of America Holdings, Medpace Holdings Inc., Parexel International Corp., PPD, Veeda Clinical Research Ltd., Vivotecnia, and WuXi AppTec Co. Ltd.
-
Market Research Insights
- The preclinical Contract Research Organization (CRO) market encompasses a diverse range of services integral to drug discovery and development. Two significant aspects of this market are bioavailability studies and safety assessment. Bioavailability studies determine the extent and rate of a lead compound's absorption into a living system, while safety assessment reports evaluate potential risks and toxicities. In 2020, the global market for bioavailability studies was estimated to be worth USD7.5 billion, with a projected CAGR of 6.3% from 2021 to 2026. In contrast, the safety assessment market was valued at USD12.8 billion in the same year, growing at a CAGR of 5.9% during the same period.
- The disparity in market size highlights the importance of both types of studies in the drug development process. Effective preclinical testing relies on meticulous experimental design, data integrity, and regulatory strategies. This includes toxicology reports, microdosing studies, and clinical pathology assessments, among others. Regulatory filings and study monitoring ensure data accuracy and adherence to ethical standards, such as animal welfare and study reporting. CROs provide crucial services in drug discovery, from target validation and drug interactions to preclinical efficacy and absorption pathways. Statistical modeling and quality control further enhance the reliability and validity of study results.
- Ultimately, the market continues to evolve, driven by the ongoing need for innovative drug development solutions.
We can help! Our analysts can customize this preclinical cro market research report to meet your requirements.