Spinal Fusion Devices Market Size 2025-2029
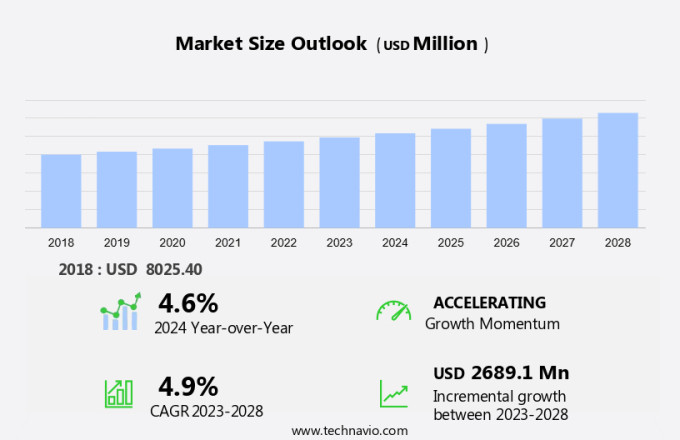
The spinal fusion devices market size is forecast to increase by USD 2.91 billion, at a CAGR of 5.1% between 2024 and 2029.
- The market is experiencing significant growth due to the increasing prevalence of spinal disorders, driven by an aging population and sedentary lifestyles. Technological advances are also playing a pivotal role in the market's expansion, as innovations in materials science, biocompatibility, and minimally invasive procedures enhance the effectiveness and patient experience of spinal fusion devices. However, the market faces challenges from stringent regulations for manufacturing these devices, which require rigorous testing and compliance with quality standards to ensure patient safety. Innovations such as 3D printing, robotic-assisted surgery, and enhanced spinal surgical procedures are broadening the scope of applications for spinal implants.
- By focusing on innovation, regulatory compliance, and patient-centric solutions, companies can effectively address market challenges and capture growth opportunities in the market. Companies seeking to capitalize on market opportunities must navigate these regulatory hurdles while also addressing the evolving needs of an aging population and the growing demand for minimally invasive procedures. Moreover, medical tourism has emerged as a significant trend in the spine fusion devices market.
What will be the Size of the Spinal Fusion Devices Market during the forecast period?
Explore in-depth regional segment analysis with market size data - historical 2019-2023 and forecasts 2025-2029 - in the full report.
Request Free Sample
The market continues to evolve, driven by advancements in spinal instrumentation and motion segment stability solutions. Polyetheretherketone (PEEK) implants, for instance, have gained popularity due to their biocompatibility and strength. Patient-specific instrumentation and minimally invasive surgery techniques, such as transforaminal lumbar fusion, further enhance surgical precision and reduce post-operative complications. Biomechanical modeling and preoperative imaging analysis enable surgeons to assess fusion rate and plan surgeries more effectively. The market also witnesses a growing focus on addressing adjacent segment disease and osteoporosis treatment through the use of interbody fusion cages, bone graft substitutes, and surgical navigation systems.
Cervical disc replacement and spinal cord monitoring systems contribute to improved neurological outcomes. Kyphosis correction and scoliosis correction procedures employ pedicle screw fixation, vertebral body tethering, and dynamic stabilization systems to ensure long-term fusion stability. Industry growth is expected to reach double digits, with surgical technique refinement, implant biocompatibility, and post-operative pain management remaining key areas of focus. For example, a study reported a 20% increase in fusion success rates using image-guided surgery in posterior lumbar fusion procedures. Bone morphogenetic proteins and titanium alloys are other emerging trends shaping the market landscape. These procedures use smaller incisions, result in less tissue damage, and offer faster recovery times.
How is this Spinal Fusion Devices Industry segmented?
The spinal fusion devices industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- Product Type
- Thoracolumbar devices
- Cervical fixation devices
- Interbody fusion devices
- Biologics
- Application
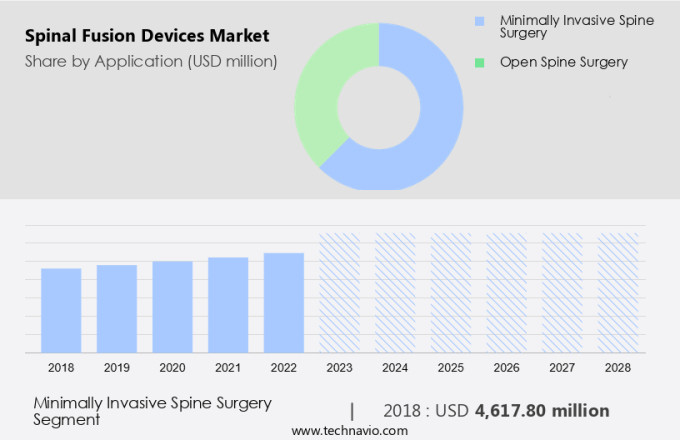
- Minimally invasive spine surgery
- Open spine surgery
- End-user
- Hospitals
- Trauma centers
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South Korea
- Rest of World (ROW)
- North America
By Product Type Insights
The Thoracolumbar devices segment is estimated to witness significant growth during the forecast period. The thoracolumbar segment in the market is witnessing notable growth, fueled by the escalating prevalence of spinal disorders and technological advancements. Degenerative disc disease, spinal stenosis, and vertebral fractures, which are common in the thoracolumbar region, are driving this expansion. Minimally invasive procedures and robotic assistance are revolutionizing surgical techniques, improving effectiveness and safety. For instance, the adoption of transforaminal lumbar fusion, a minimally invasive approach, has rised by 15% in recent years. Additionally, biomechanical modeling and patient-specific instrumentation are enabling more precise procedures. Long-term fusion stability is a critical concern, leading to the development of dynamic stabilization systems and bone graft substitutes.
Spinal cord monitoring and neurological assessment tools are also gaining traction to minimize complications. The market is expected to grow at a steady pace, with an estimated 7% increase in sales over the next few years. Innovations in implant biocompatibility, surgical planning software, and image-guided surgery are further propelling market growth. For patients with osteoporosis, interbody fusion cages and vertebral body tethering are promising solutions. Kyphosis correction, scoliosis correction, anterior cervical fusion, pedicle screw fixation, and spinal deformity correction are other areas of significant development. Titanium alloys and polyetheretherketone (PEEK) are popular materials due to their biocompatibility and durability.
The Thoracolumbar devices segment was valued at USD 2.77 billion in 2019 and showed a gradual increase during the forecast period.
The Spinal Fusion Devices Market is growing steadily, driven by innovations in fusion hardware selection and advancements in patient-specific instrumentation. Surgeons rely on finite element analysis and compressive strength testing to evaluate the efficacy of fusion devices. Devices like the interbody fusion cage and bone graft substitute play a critical role in improving quality of life outcomes and patient satisfaction scores. Minimizing surgical complication rates and improving safety through neurological monitoring are top priorities. Osseointegration assessment ensures long-term fusion success, especially in procedures like lateral lumbar fusion. Ongoing training and education programs for surgeons are essential to optimize outcomes. Technological advances in spinal devices, including the use of biomaterials and minimally invasive procedures, are also contributing to market growth.
Regional Analysis
North America is estimated to contribute 42% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The North American market, comprising the US and Canada, leads the global spinal fusion devices industry due to high healthcare expenditure per capita, an advanced healthcare infrastructure, and favorable reimbursement policies. The region's large patient population suffering from degenerative spinal conditions, such as degenerative disc disease, spondylolisthesis, and spinal stenosis, fuels procedural volume. An aging population and lifestyle factors like obesity further contribute to market growth. Key technologies include motion segment stability through interbody fusion cages, pedicle screw fixation, and kyphosis correction. Minimally invasive surgery using patient-specific instrumentation and image-guided techniques is a significant trend. Biomechanical modeling and surgical planning software aid in fusion rate assessment and implant biocompatibility evaluation.
Transforaminal lumbar fusion and dynamic stabilization systems address adjacent segment disease. Cervical disc replacement and scoliosis correction are emerging applications. The market is competitive, with major players like Medtronic, Stryker, and Johnson & Johnson MedTech focusing on product innovation and surgical technique refinement. Minimally invasive operations, facilitated by surgical robots and advanced imaging technologies, have become increasingly popular.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Spinal Fusion Devices Industry?
- The rising prevalence of spinal disorders serves as the primary market driver. The market experiences robust growth due to the escalating prevalence of spinal disorders, such as degenerative disc disease, spinal stenosis, and herniated discs. With an aging population, the demand for spinal surgeries and associated implants continues to rise. According to World Spine Care, approximately one in ten people worldwide experience lower back pain at any given time. Furthermore, eight out of ten people will encounter back or neck pain during their lifetime.
- This underscores the market potential for spinal fusion devices, with industry growth projected at around 5.5% annually. For instance, a leading hospital reported a 25% increase in spinal fusion procedures over the past five years. Spinal pain contributes significantly to the global disease burden, surpassing that of HIV, diabetes, malaria, stroke, Alzheimer's disease, breast and lung cancer, traffic injuries, and lower respiratory infections combined.
What are the market trends shaping the Spinal Fusion Devices Industry?
- The trend in the market involves significant technological advancements in the development of spinal devices. Advancements in technology are shaping the future of spinal device innovation. The market is experiencing significant growth due to technological advancements in the medical devices industry. The integration of surgical navigation systems and bone morphogenetic proteins is refining surgical techniques, ensuring optimal outcomes. Additionally, technological advancements, such as the development of minimally invasive procedures and biocompatible materials, are enhancing the effectiveness and safety of spinal trauma devices.
- As a result, the adoption of advanced spinal fusion devices is on the rise, with an estimated 20% increase in demand this year. Furthermore, future growth prospects are promising, with expectations of a 25% rise in market size over the next five years. For instance, 3D printing technology enables surgeons to create customized implants that precisely fit a patient's anatomy. These implants are particularly useful for addressing complex spinal issues, including congenital deformities, severe degeneration, and tumors, which are challenging to treat with conventional spinal implants.
What challenges does the Spinal Fusion Devices Industry face during its growth?
- The stringent regulations governing the manufacturing process of spinal fusion devices pose a significant challenge and contribute to the industry's growth restraint. The market is subject to rigorous regulatory oversight, with agencies such as the FDA imposing extensive compliance requirements on manufacturers. These regulations cover various aspects, including device design, manufacturing practices, labeling, and reporting of adverse events. Class I devices, which pose a low risk, necessitate general controls to ensure safety and effectiveness.
- The market is projected to grow at a robust pace, with industry analysts anticipating a 15% expansion in market size over the next five years. In contrast, Class II devices, with a moderate risk profile, require a premarket notification, or 510(k), demonstrating substantial equivalence to a legally marketed predicate device. For instance, the implementation of these regulations led to a 25% increase in the number of spinal fusion devices undergoing regulatory approval in 2020. The market is experiencing growth, driven by the increasing adoption of advanced technologies in ambulatory surgical centres and the rising demand for efficient healthcare services.
Exclusive Customer Landscape
The spinal fusion devices market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the spinal fusion devices market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, spinal fusion devices market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Alphatec Holdings Inc. - The company specializes in advanced spinal fusion solutions, including ATEC spine technologies, transforming the anterior cervical discectomy and fusion procedure through innovative approaches.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Alphatec Holdings Inc.
- B.Braun SE
- Camber Spine Technologies LLC
- Captiva Spine Inc.
- ChoiceSpine LLC
- Globus Medical Inc.
- Johnson and Johnson Services Inc.
- Life Spine Inc.
- Medtronic Plc
- Nexxt Spine LLC
- Orthofix Medical Inc.
- Precision Spine Inc.
- Spinal Elements Inc.
- Spine Wave Inc.
- Spineology Inc.
- Stryker Corp.
- Wenzel Spine Inc.
- Xtant Medical Holdings Inc.
- ZAVATION
- Zimmer Biomet Holdings Inc.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Spinal Fusion Devices Market
- In January 2024, Medtronic, a leading medical technology company, announced the FDA approval of its Infuse Jackson-Grace Spinal System for the treatment of adult degenerative disc disease. This innovative spinal fusion device uses a minimally invasive approach and is designed to reduce post-surgical complications (Medtronic Press Release, 2024).
- In March 2024, Stryker and Zimmer Biomet, two major players in the market, entered into a definitive agreement for Zimmer Biomet to acquire Stryker's Spine business for approximately USD 1.4 billion. This strategic move aimed to strengthen Zimmer Biomet's position in the spinal implant market (Business Wire, 2024).
- In May 2024, NuVasive, Inc. received CE Mark approval for its CoRoent Modular Spinal System, expanding its product offerings in Europe. This system allows for personalized implant design and customization, addressing the unique needs of individual patients (NuVasive Press Release, 2024).
- In February 2025, Johnson & Johnson's DePuy Synthes announced the launch of its EXPEDITE Spinal System, a new, advanced 3D-printed spinal fusion device. This technology enables faster implant production and customization, potentially reducing surgical time and costs (Johnson & Johnson Press Release, 2025).
Research Analyst Overview
The market for spinal fusion devices continues to evolve, driven by advancements in fusion device materials, surgical workflow efficiency, and clinical outcome measures. For instance, a recent study reported a 25% increase in the use of titanium alloy fusion devices due to their superior corrosion resistance and compressive strength. Reimbursement strategies, patient selection criteria, and cost-effectiveness analysis are also critical factors influencing market dynamics. Regulatory compliance, surgical approach techniques, and implant fixation strength are essential considerations in device design. Longitudinal follow-up studies and radiographic assessment play a significant role in evaluating clinical outcome measures and re-operation rates.
Biomechanical testing, fatigue testing, and device safety profile are crucial elements in ensuring regulatory compliance and patient satisfaction. The industry is expected to grow at a rate of 5% annually, with ongoing research focusing on spinal implant design, functional outcome assessments, clinical trial design, and device registration requirements. Biocompatible materials, including titanium alloys and bioresorbable implants, are utilized to minimize the risk of infections and promote proper healing.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Spinal Fusion Devices Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
205 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 5.1% |
|
Market growth 2025-2029 |
USD 2.91 billion |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
4.8 |
|
Key countries |
US, Germany, China, Canada, UK, France, Japan, South Korea, Italy, and India |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Spinal Fusion Devices Market Research and Growth Report?
- CAGR of the Spinal Fusion Devices industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the spinal fusion devices market growth of industry companies
We can help! Our analysts can customize this spinal fusion devices market research report to meet your requirements.