Covid-19 Vaccination Market 2024-2028
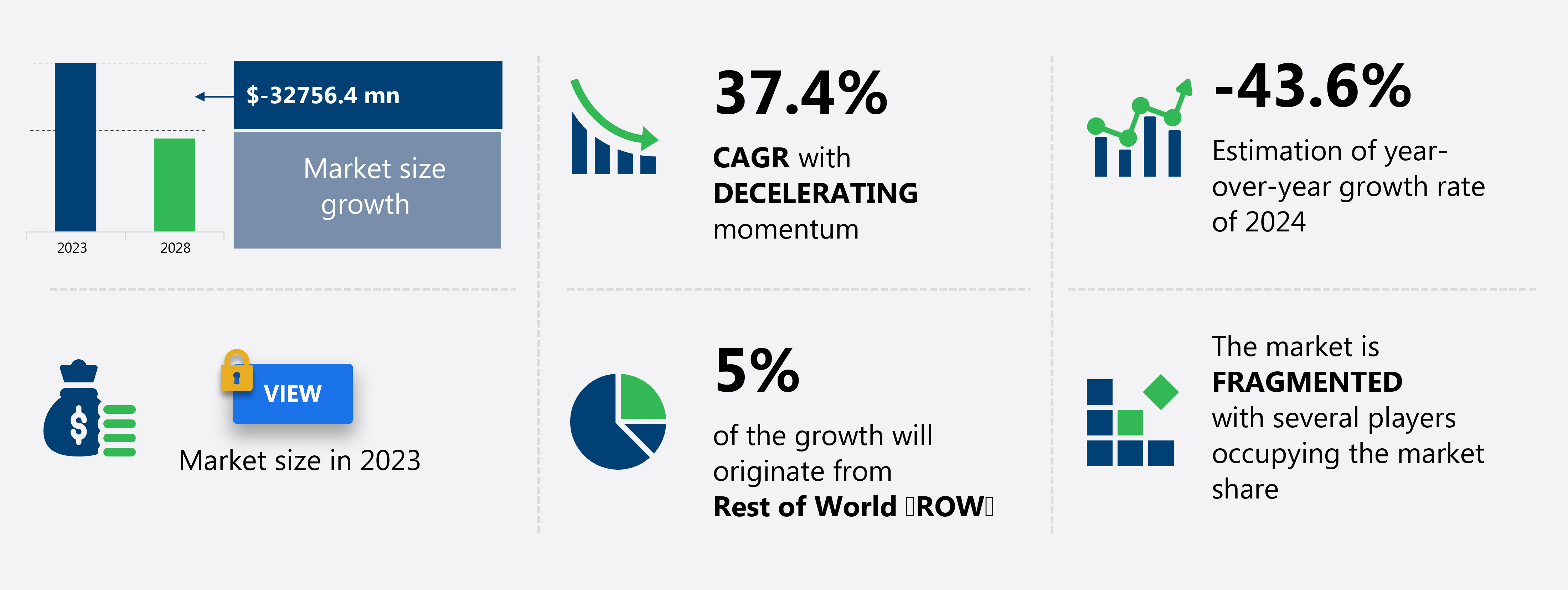
The covid-19 vaccination market size is forecast to increase by USD -32.76 billion, at a CAGR of -37.4% between 2023 and 2028. The market is experiencing significant growth due to the expansion of vaccination programs worldwide. Governments and international organizations are investing heavily in vaccination initiatives to contain the spread of the virus. The rising research and development (R&D) investment in the development of Covid-19 vaccines is another major growth factor. However, the high cost of production of Covid-19 vaccines poses a significant challenge to market growth. Manufacturers are exploring various strategies to reduce production costs while maintaining vaccine efficacy and safety. The market is expected to witness strong growth in the coming years as more effective and affordable vaccines become available. poiuyfrtyh
What will the Covid-19 Vaccination Market Size be During the Forecast Period?
Download Report Sample to Unlock the Covid-19 Vaccination Market Size for the Forecast Period and Other Important Statistics
Market Dynamics
The COVID-19 pandemic has brought about an unprecedented global health crisis, leading to the development of numerous vaccines to mitigate its impact. This content focuses on various aspects of COVID-19 vaccines, including production, distribution, administration, efficacy, safety, and regulations. COVID-19 vaccine production has been a top priority for researchers and pharmaceutical companies worldwide. Several manufacturers have developed vaccines using various technologies such as mRNA, viral vector, and protein subunit, undergoing rigorous testing and clinical trials to ensure safety and efficacy. Once vaccines receive approval from regulatory bodies, they are distributed to healthcare facilities and vaccination centers, requiring careful planning and coordination. Governments and international organizations are working to ensure equitable distribution, prioritizing vulnerable populations and herd immunity. Vaccine administration involves healthcare professionals delivering vaccines through injections, with proper training and safety protocols to minimize adverse reactions. Efficacy refers to the vaccine's ability to prevent infection or reduce the severity of symptoms, with most vaccines showing high efficacy rates, ranging from 60% to 95%. Vaccine safety is monitored closely, and while common side effects include pain and swelling at the injection site, fever, and fatigue, serious side effects are rare.
Vaccine procurement involves purchasing vaccines from manufacturers, with governments securing supplies through contracts and partnerships. Vaccine allocation ensures that vaccines are distributed to specific populations, with priority given to vulnerable groups like healthcare workers and the elderly. Vaccine prioritization determines which populations should receive vaccines first, based on risk factors. Vaccine passports are digital or physical documents that prove vaccination status, and may be required for travel or work, with regulations varying by jurisdiction. Vaccine mandates, which require vaccination for employment or participation in certain activities, remain a controversial issue. Vaccine regulations ensure vaccines are safe and effective, and policies governing vaccine use in schools, workplaces, and travel may change as supplies and public health conditions evolve.
Covid-19 Vaccination Market Driver
The expansion of vaccination programs is the key driver of the market. The market is experiencing significant growth due to the increasing demand for vaccines as governments and healthcare organizations prioritize widespread vaccination to control the virus and achieve herd immunity. This heightened demand leads to increased production and sales for vaccine manufacturers, resulting in long-term procurement contracts being signed to ensure a consistent vaccine supply. These contracts provide stability and revenue for manufacturers, with more contracts expected to be established as vaccination programs expand.
Vaccine distribution, administration, and logistics are crucial elements in the vaccine market, requiring efficient vaccine storage, transportation, and scheduling. Vaccine safety, efficacy, and monitoring are also vital considerations, along with addressing vaccine hesitancy and acceptance through education and outreach efforts. Vaccine regulations, policies, and campaigns are essential in ensuring vaccine coverage, immunity, and compliance with side effects and potential mandates or certificates.
Covid-19 Vaccination Market Trends
Rising research and development investment is the upcoming trend in the market. The Covid-19 pandemic has necessitated the rapid development, production, and distribution of vaccines to prevent and treat the disease caused by the SARS-CoV-2 virus. Governments and the private sector have collaborated to invest in vaccine research and development. In May 2020, the US Department of Health and Human Services launched "Operation Warp Speed," a collaborative initiative involving the CDC, FDA, NIH, and Department of Defense, with funding from the Biomedical Advanced Research and Development Authority (BARDA). This program has provided over USD 19 billion in assistance to seven pharmaceutical manufacturers to expedite vaccine production. Vaccine distribution, administration, and rollout have been prioritized globally to contain the pandemic.
Vaccine safety, efficacy, and monitoring are critical aspects of the vaccine development process. Vaccine logistics, including storage, transportation, and supply chain management, are essential to ensure vaccine doses reach those in need. Vaccine manufacturers and suppliers have been working to increase production capacity and ensure a steady supply of doses. Vaccine acceptance and coverage have been influenced by factors such as vaccine hesitancy, side effects, and regulations. Vaccine policies, campaigns, education, and outreach efforts have been implemented to address these concerns and increase vaccine acceptance. Vaccine passports, certificates, and mandates have been proposed as potential solutions to encourage vaccination and facilitate economic recovery.
Covid-19 Vaccination Market Challenge
The high cost of production of COVID-19 vaccines is a key challenge affecting the market growth. The market encompasses various aspects, including vaccine distribution, administration, production, and development. The cost of creating a single epidemic infectious disease vaccine, from preclinical trials through Phase IIa, ranges from USD 31-USD 68 million, assuming no clinical trial failures. Advancing at least one vaccine to the end of Phase IIa trials incurs an average cost of USD 84 to USD 112 million. Vaccine manufacturing facilities entail substantial fixed and ongoing maintenance costs, ranging from USD 50 to USD 500 million per antigen, contingent on automation and design complexity. Vaccine distribution, administration, and rollout involve intricate logistics, including vaccine storage, transportation, and supply chain management. Vaccine efficacy and safety are crucial factors in vaccine development and approval, necessitating rigorous clinical trials and regulatory compliance.
Vaccine procurement, allocation, prioritization, and scheduling are essential components of vaccine deployment. Vaccine monitoring, side effects, hesitancy, and acceptance are significant considerations in vaccine campaigns and outreach efforts. Vaccine immunity, certificates, passports, mandates, regulations, and policies are topics of ongoing debate and discussion. Vaccine education and awareness are vital to ensuring broad population coverage and herd immunity.
Who are the Major Covid-19 Vaccination Market Companies?
Companies are implementing various strategies, such as strategic alliances, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the market.
AIVITA Biomedical Inc. - The company offers COVID-19 vaccines, including Vaxzevria, for primary immunization and initial booster doses in accordance with health authorities' guidelines.
The research report also includes detailed analyses of the competitive landscape of the market and information about 20 market companies, including:
- AnGes Inc.
- AstraZeneca Plc
- Bharat Biotech Ltd.
- CureVac AG
- Emergent BioSolutions Inc.
- EuBiologics Co. Ltd.
- Genexine Inc.
- GreenLight Biosciences Holdings PBC
- Inovio Pharmaceuticals Inc.
- Johnson and Johnson Services Inc.
- Moderna Inc.
- Novavax Inc.
- Pfizer Inc.
- Serum Institute of India Pvt. Ltd.
- Shenzhen Kangtai Biological Products Co. Ltd.
- Sinovac Biotech Ltd.
- Tadbir innovation pharmaceutical Co.
- The Gamaleya National Center of Epidemiology and Microbiology
- Zydus Lifesciences Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key market players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Which are the Key Regions for the Market?
For more insights on the market share of various regions Request PDF Sample now!
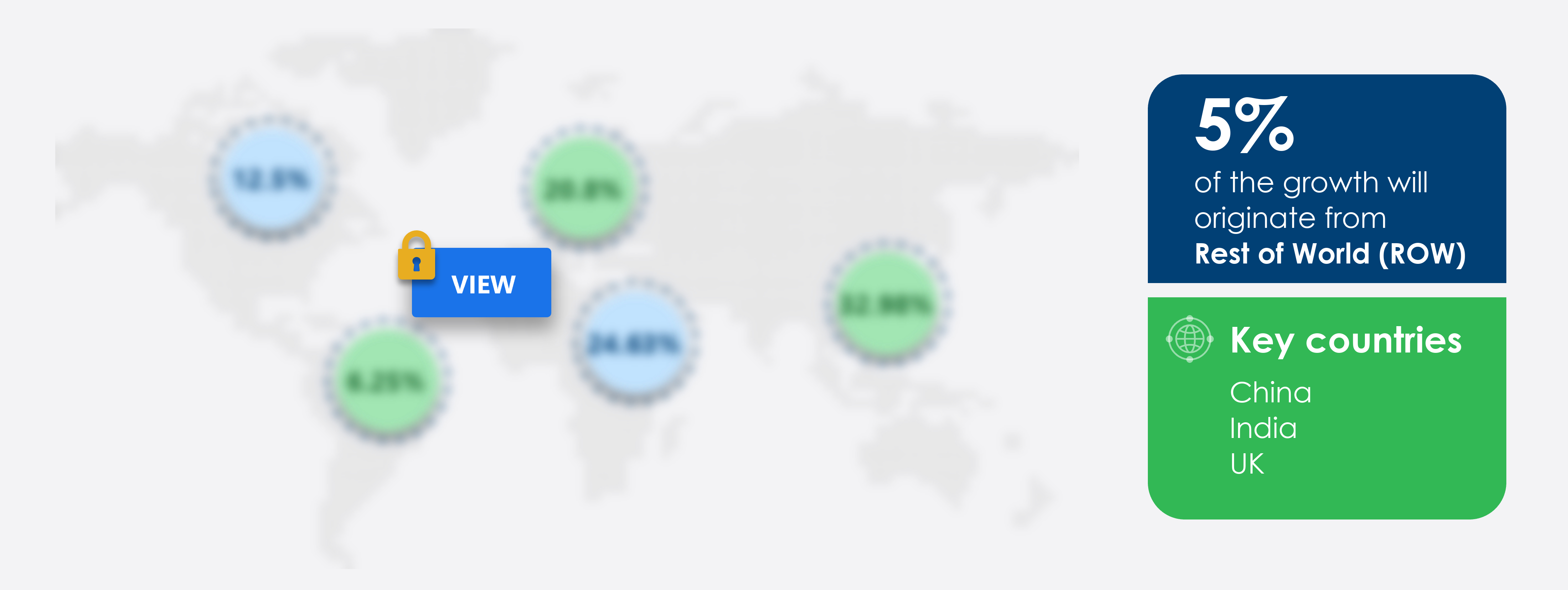
Rest of World (ROW) is estimated to contribute 5% to the growth of the global market during the forecast period. Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period. The market is witnessing significant growth due to the global effort to combat the ongoing pandemic. Vaccine efficacy rates continue to be a topic of interest, with some vaccines demonstrating high efficacy rates ranging from 60% to 95%. Vaccine booster shots are being considered to enhance vaccine protection, particularly against emerging vaccine variants. The duration of vaccine immunity is also a critical factor, with ongoing research to determine how long immunity lasts and whether booster shots will be necessary. Vaccine protection against new variants remains a key challenge, with ongoing monitoring and potential adjustments to vaccines necessary to maintain effectiveness.
What are the Revenue-generating Distribution Channel Segments in the Market?
To gain further insights on the market contribution of various segments Request a PDF Sample
The government entities segment is estimated to witness significant growth during the forecast period. The market encompasses the production, distribution, administration, and monitoring of vaccines to combat the global pandemic. Vaccine development and research have been the focus of various organizations and governments worldwide, with numerous vaccine trials underway to assess vaccine efficacy and safety. Vaccine approval is a critical step in the vaccine rollout, with regulatory bodies evaluating data from clinical trials and ensuring adherence to safety standards. Vaccine supply chain and logistics are essential components of the vaccination market, ensuring the timely and efficient delivery system of vaccine doses. Vaccine storage and transportation require specialized infrastructure to maintain the required temperature and conditions.
Market Analyst Overview
The market has experienced unprecedented growth and global attention since the onset of the pandemic. Vaccine development, production, and distribution have been prioritized by governments and international organizations to combat the spread of the virus. Vaccine trials and approval processes have been expedited, with several vaccines demonstrating high efficacy and safety. Vaccine manufacturers and suppliers have ramped up production to meet the demand for billions of doses. Vaccine logistics, including storage, transportation, and distribution, have been critical in ensuring the successful rollout of vaccines. Vaccine allocation, prioritization, and scheduling have been key considerations in vaccine deployment. Vaccine monitoring and surveillance systems have been put in place to track vaccine efficacy, safety, and side effects.
Vaccine acceptance and coverage have been influenced by factors such as vaccine hesitancy, education, and outreach efforts. Vaccine regulations, policies, and campaigns have been implemented to address these challenges and promote vaccine uptake. Vaccine certificates and passports have emerged as potential solutions to facilitate travel and ease restrictions in some areas. Despite the progress made in vaccine development and distribution, challenges remain in ensuring equitable access to vaccines, addressing vaccine hesitancy, and ensuring the safe and efficient distribution of vaccines to those in need. Ongoing research and collaboration between governments, organizations, and the private sector will be essential in addressing these challenges and ensuring the successful implementation of COVID-19 vaccines.
|
Covid-19 Vaccination Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
120 |
|
Base year |
2023 |
|
Forecast period |
2024-2028 |
|
Growth momentum & CAGR |
Decelerate at a CAGR of -37.4% |
|
Market Growth 2024-2028 |
USD -32.76 billion |
|
Market structure |
Fragmented |
|
YoY growth (%) |
-43.6 |
|
Regional analysis |
North America, Asia, Europe, and Rest of World (ROW) |
|
Performing market contribution |
Rest of World (ROW) at 5% |
|
Key consumer countries |
China, India, UK, France, and US |
|
Competitive landscape |
Leading companies, Competitive Strategies, Consumer engagement scope |
|
Key companies profiled |
AIVITA Biomedical Inc., AnGes Inc., AstraZeneca Plc, Bharat Biotech Ltd., CureVac AG, Emergent BioSolutions Inc., EuBiologics Co. Ltd., Genexine Inc., GreenLight Biosciences Holdings PBC, Inovio Pharmaceuticals Inc., Johnson and Johnson Services Inc., Moderna Inc., Novavax Inc., Pfizer Inc., Serum Institute of India Pvt. Ltd., Shenzhen Kangtai Biological Products Co. Ltd., Sinovac Biotech Ltd., Tadbir innovation pharmaceutical Co., The Gamaleya National Center of Epidemiology and Microbiology, and Zydus Lifesciences Ltd. |
|
Market dynamics |
Parent market growth analysis, Market research and growth, Market growth and forecasting, Market forecasting, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, COVID-19 impact and recovery analysis and future consumer dynamics, Market condition analysis for the market forecast period |
|
Customization purview |
If our report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this Covid-19 Vaccination Market Report?
- CAGR of the market during the forecast period 2024-2028
- Detailed information on factors that will drive covid-19 vaccination market growth during the next five years
- Precise estimation of the covid-19 vaccination market size and its contribution to the parent market
- Accurate predictions on upcoming trends and changes in consumer behavior
- The growth of the covid-19 vaccination market industry across North America, Asia, Europe, and Rest of World (ROW)
- A thorough analysis of the market's competitive landscape and detailed information on vendors
- Comprehensive details of factors that will challenge the growth of covid-19 vaccination market vendors
We can help! Our analysts can customize this report to meet your requirements.