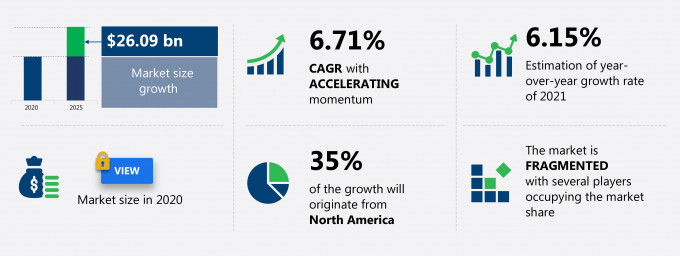
The clinical reference laboratory services market share is expected to increase by USD 26.09 billion from 2020 to 2025, and the market’s growth momentum will accelerate at a CAGR of 6.71%.
This clinical reference laboratory services market research report provides valuable insights on the post COVID-19 impact on the market, which will help companies evaluate their business approaches. Furthermore, this report extensively covers clinical reference laboratory services market segmentations by end-user (hospitals and private clinics, corporate offices and companies, and government entities), service (stand-alone reference laboratories, hospital-based reference laboratories, and clinic-based reference laboratories), and geography (North America, APAC, Europe, South America, and MEA). The clinical reference laboratory services market report also offers information on several market vendors, including amedes Holding GmbH, Clinical Reference Laboratory Inc., Exact Sciences Corp., Mayo Medical Laboratories, OPKO Health Inc., Quest Diagnostics Inc., Sonic Healthcare Ltd., SYNLAB Bondco Plc, and Unilabs AB among others.
What will the Clinical Reference Laboratory Services Market Size be During the Forecast Period?
Download the Free Report Sample to Unlock the Clinical Reference Laboratory Services Market Size for the Forecast Period and Other Important Statistics
Clinical Reference Laboratory Services Market: Key Drivers, Trends, and Challenges
The increasing prevalence of chronic and infectious diseases is notably driving the clinical reference laboratory services market growth, although factors such as stringent regulations may impede market growth. Our research analysts have studied the historical data and deduced the key market drivers and the COVID-19 pandemic impact on the clinical reference laboratory services market industry. The holistic analysis of the drivers will help in deducing end goals and refining marketing strategies to gain a competitive edge.
Key Clinical Reference Laboratory Services Market Driver
One of the key factors driving the clinical reference laboratory services market is the increasing prevalence of chronic and infectious diseases. Medical diagnostic procedures are in high demand due to the rising prevalence of chronic and infectious diseases. Furthermore, reference laboratories are providing services for the rapid detection of infectious diseases that require specialized laboratory testing instruments and skills, such as swine flu, hepatitis A and B, and HIV. These labs also provide low-cost diagnostic tests for common chronic diseases like cancer, diabetes, and cardiovascular disease. Furthermore, during peak seasons of infection, the lack of expertise in hospitals and clinics to perform critical diagnostic testing procedures for pandemic and highly communicable diseases, such as swine flu and Zika virus infection, necessitates the outsourcing of these tests to specialized reference laboratories. According to the World Bank Group, 1.4 million people die each year from hepatitis virus infections of various types. Hepatitis B vaccination based on blood test results has increased over time, according to the CDC, from 12.3% in 1999-2002 to 25.2% in 2015-2018. Furthermore, the CDC estimates that since 2010, influenza has caused 9-45 million cases, 140,000-810,000 hospitalizations, and 12,000-61,000 deaths. As a result, the global clinical reference laboratory services market will continue to grow during the forecast period due to the rising prevalence of chronic and infectious diseases.
Key Clinical Reference Laboratory Services Market Trend
Increasing partnerships between reference laboratories and risk management service providers is another factor supporting the clinical reference laboratory services market growth in the forecast period. Vendors operating at the global level face stiff competition from local vendors in terms of customer acquisition. Therefore, to overcome such issues, established players are focusing on collaborating with entities such as health plan providers, integrated delivery network (IDN) and risk-bearing entities through partnerships. These partnerships enable reference laboratories to maintain a reliable customer base, accelerate growth, and achieve operational excellence. Moreover, these partnerships also allow the transparent flow of information and unrestricted sharing of data to empower healthcare services by utilizing advanced IT structures and providing efficient diagnostic insights. In July 2019, Bioreference Laboratories entered into a strategic collaboration with SOMOS, a physician network led by more than 2,500 healthcare providers serving over 700,000 Medicaid beneficiaries in New York, US. This collaboration has helped OPKO Health to become a preferred provider for diagnostic testing and assist patients with data analytics. Such partnerships allow vendors to retain their customer base by attaining the preferred laboratory status for clinical testing. This has encouraged the established reference laboratories to plan for long-term strategies to offer cost-effective testing services in the market and overcome the challenges posed by local vendors.
Key Clinical Reference Laboratory Services Market Challenge
Stringent regulations will be a major challenge for the clinical reference laboratory services market during the forecast period. Vendor-provided laboratory testing services are influenced by or subjected to complex and frequently changing laws and regulations around the world. Vendors' ability to operate their businesses quickly in a competitive market is hampered by frequent changes in laws. Furthermore, reference tests are important because they provide results for clinical diagnostic testing, and test results that are inaccurate can have serious and life-threatening consequences for patients' health. As a result, regulatory agencies all over the world have put in place strict regulations and defined standards. CLIA, for example, oversees the operation of clinical laboratories in the United States, ensuring that they meet various operational, technical, quality, and personnel requirements in order to provide reliable, accurate, and timely service. Similarly, the FDA in the US regulates devices, instruments, kits, software, and reagents used in reference test services. Similarly, in Europe, laboratory testing is governed by the European Parliament and Council Regulation (EC) No 882/2004 on official controls. The EU requires national reference laboratories to operate in accordance with EN ISO/EN 17011 and EN ISO/IEC 17025, which are updated on a regular basis. By limiting the operational capacities of reference laboratories, these standards and regulations limit the market's growth.
This clinical reference laboratory services market analysis report also provides detailed information on other upcoming trends and challenges that will have a far-reaching effect on the market growth. The actionable insights on the trends and challenges will help companies evaluate and develop growth strategies for 2021-2025.
Parent Market Analysis
Technavio categorizes the global clinical reference laboratory services market as a part of the global life sciences tools and services market. Our research report has extensively covered external factors influencing the parent market growth potential in the coming years, which will determine the levels of growth of the clinical reference laboratory services market during the forecast period.
Who are the Major Clinical Reference Laboratory Services Market Vendors?
The report analyzes the market’s competitive landscape and offers information on several market vendors, including:
- amedes Holding GmbH
- Clinical Reference Laboratory Inc.
- Exact Sciences Corp.
- Mayo Medical Laboratories
- OPKO Health Inc.
- Quest Diagnostics Inc.
- Sonic Healthcare Ltd.
- SYNLAB Bondco Plc
- Unilabs AB
This statistical study of the clinical reference laboratory services market encompasses successful business strategies deployed by the key vendors. The clinical reference laboratory services market is fragmented and the vendors are deploying growth strategies such as collaborations between end-users and established vendors to compete in the market.
Product Insights and News
- amedes Holding GmbH - The company offers clinical laboratory services to Porphyria, Allergy, Fat metabolism, Hematology and others.
- Mayo Medical Laboratories - The company offers clinical testing for Allergens, Endocrinology, Cardiology, Fertility, Oncology, Renal and others.
To make the most of the opportunities and recover from post COVID-19 impact, market vendors should focus more on the growth prospects in the fast-growing segments, while maintaining their positions in the slow-growing segments.
The clinical reference laboratory services market forecast report offers in-depth insights into key vendor profiles. The profiles include information on the production, sustainability, and prospects of the leading companies.
Clinical Reference Laboratory Services Market Value Chain Analysis
Our report provides extensive information on the value chain analysis for the clinical reference laboratory services market, which vendors can leverage to gain a competitive advantage during the forecast period. The end-to-end understanding of the value chain is essential in profit margin optimization and evaluation of business strategies. The data available in our value chain analysis segment can help vendors drive costs and enhance customer services during the forecast period.
The value chain of global life sciences tools and services market includes the following core components:
- Inputs
- Inbound logistics
- Operations
- Outbound logistics
- Marketing and sales
- Service
- Support activities
- Innovation
The report has further elucidated on other innovative approaches being followed by service providers to ensure a sustainable market presence.
Which are the Key Regions for Clinical Reference Laboratory Services Market?
For more insights on the market share of various regions Request for a FREE sample now!
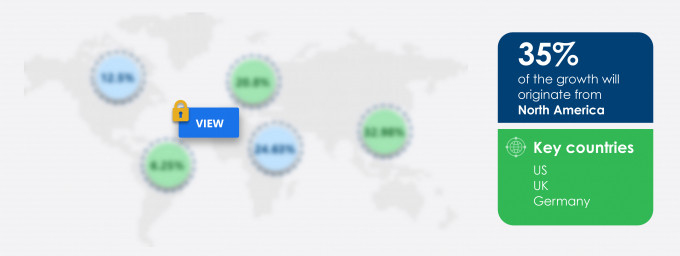
35% of the market’s growth will originate from North America during the forecast period. The US is one of the key markets for clinical reference laboratory services market in North America. Market growth in this region will be slower than the growth of the market in Asia.
The increasing number of end-users because of the rising incidence of health conditions is one of the prime factors that will facilitate the clinical reference laboratory services market growth in North America over the forecast period. This market research report entails detailed information on the competitive intelligence, marketing gaps, and regional opportunities in store for vendors, which will assist in creating efficient business plans.
COVID Impact and Recovery Analysis
The clinical reference laboratory services market in North America is expected to witness strong growth during 2020-2021, owing to the outbreak of COVID-19. The rising number of COVID-19 cases has significantly increased the pressure on healthcare facilities in this region. Laboratories are continually increasing their testing capacities to address the rising demand for COVID-19 testing. Such increased demand for COVID-19 testing is expected to facilitate the growth of the clinical reference laboratory services market in the region during the forecast period.
What are the Revenue-generating End-user Segments in the Clinical Reference Laboratory Services Market?
To gain further insights on the market contribution of various segments Request for a FREE sample
The market share growth by the end-user segments. Hospitals and private clinics segment will be significant during the forecast period. Hospitals and clinics outsource a significant number of clinical testing procedures that cannot be performed in in-house laboratories. Hence, the hospitals and private clinics segment is the major revenue-contributing end-user segment of the market. Hospital budgets are rapidly constraining, owing to the lack of funding and declining reimbursement coverage. The reducing cost of laboratory expenditure in hospitals is a primary driver for the outsourcing of laboratory services.
This report provides an accurate prediction of the contribution of all the segments to the growth of the clinical reference laboratory services market size and actionable market insights on post COVID-19 impact on each segment.
|
Clinical Reference Laboratory Services Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
120 |
|
Base year |
2020 |
|
Forecast period |
2021-2025 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 6.71% |
|
Market growth 2021-2025 |
$ 26.09 billion |
|
Market structure |
Fragmented |
|
YoY growth (%) |
6.15 |
|
Regional analysis |
North America, APAC, Europe, South America, and MEA |
|
Performing market contribution |
North America at 35% |
|
Key consumer countries |
US, UK, Germany, France, and Japan |
|
Competitive landscape |
Leading companies, Competitive strategies, Consumer engagement scope |
|
Key companies profiled |
amedes Holding GmbH, Clinical Reference Laboratory Inc., Exact Sciences Corp., Mayo Medical Laboratories, OPKO Health Inc., Quest Diagnostics Inc., Sonic Healthcare Ltd., SYNLAB Bondco Plc, and Unilabs AB |
|
Market dynamics |
Parent market analysis, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, COVID 19 impact and recovery analysis and future consumer dynamics, Market condition analysis for forecast period |
|
Customization purview |
If our report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this Clinical Reference Laboratory Services Market Report?
- CAGR of the market during the forecast period 2021-2025
- Detailed information on factors that will drive clinical reference laboratory services market growth during the next five years
- Precise estimation of the clinical reference laboratory services market size and its contribution to the parent market
- Accurate predictions on upcoming trends and changes in consumer behavior
- The growth of the clinical reference laboratory services industry across North America, APAC, Europe, South America, and MEA
- A thorough analysis of the market’s competitive landscape and detailed information on vendors
- Comprehensive details of factors that will challenge the growth of clinical reference laboratory services market vendors
We can help! Our analysts can customize this report to meet your requirements. Get in touch