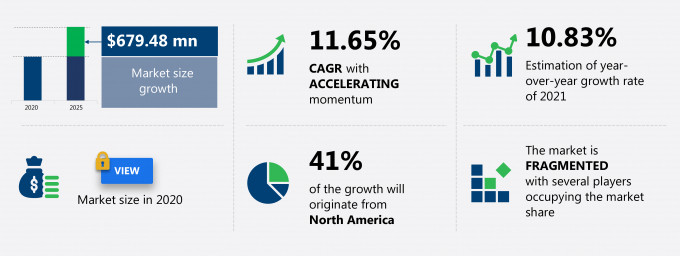
The electronic trial master file systems market share is expected to increase by USD 679.48 million from 2020 to 2025, and the market’s growth momentum will accelerate at a CAGR of 11.65%.
This electronic trial master file systems market research report provides valuable insights on the post COVID-19 impact on the market, which will help companies evaluate their business approaches. Furthermore, this report extensively covers electronic trial master file systems market segmentation by deployment (on-premise and cloud) and geography (North America, Europe, APAC, MEA, and South America). The electronic trial master file systems market report also offers information on several market vendors, including Aurea Corp., IQVIA Holdings Inc., Laboratory Corp. of America Holdings, MasterControl Inc., Oracle Corp., Phlexglobal Ltd., SureClinical Inc., TransPerfect Global Inc., Veeva Systems Inc., and WIRB Copernicus Group among others.
What will the Electronic Trial Master File Systems Market Size be During the Forecast Period?
Download the Free Report Sample to Unlock the Electronic Trial Master File Systems Market Size for the Forecast Period and Other Important Statistics
Electronic Trial Master File Systems Market: Key Drivers, Trends, and Challenges
The increasing number of clinical trials is notably driving the electronic trial master file systems market growth, although factors such as budget constraints may impede the market growth. Our research analysts have studied the historical data and deduced the key market drivers and the COVID-19 pandemic impact on the electronic trial master file systems industry. The holistic analysis of the drivers will help in deducing end goals and refining marketing strategies to gain a competitive edge.
Key Electronic Trial Master File Systems Market Driver
One of the key factors driving growth in the electronic trial master file systems market is the increasing number of clinical trials. The number of clinical trials has increased significantly worldwide, with an average annual increase in the number of registered trials being generally higher in Asia than in the US and the EU. Currently, in Asian countries, such as Japan and India, the annual number of registered clinical trials has increased progressively, owing to the implementation of numerous local reforms that have encouraged and enforced registration. The availability of a skilled workforce, favorable market reforms, access to the fastest-growing pharmaceutical markets in the world, strong intellectual property laws, and improved infrastructure for clinical trials have contributed to an increase in the number of clinical trials in developing countries. Also, the outbreak of COVID-19 has led to a rapid surge in clinical trials for drugs across various parts of the globe.
Key Electronic Trial Master File Systems Market Trend
The rising adoption of Electronic Trial Master File (eTMF) systems in developing countries is another factor supporting the electronic trial master file systems market share growth. The rapid development of COVID-19 vaccines may lead more drug developers in developing countries to increase their speed and agility by adopting electronic Trial Master File (eTMF) systems. These eTMF systems manage critical documents related to a clinical trial. In the case of COVID-19 vaccines, eTMF systems play a role in their safe acceleration. They are built to reduce human errors when managing the trial master file. Thus, the growing clinical trials for COVID-19 vaccines and treatment drugs in developing countries are leading to an increase in the adoption of eTMF and thus driving the growth of the market in focus.
Key Electronic Trial Master File Systems Market Challenge
The budget constraints will be a major challenge for the electronic trial master file systems market during the forecast period. The management of essential clinical trial documentation is one of the most time-consuming and costly activities associated with conducting a clinical trial. The ICH E6 guidance on Good Clinical Practice (GCP) specifies an inventory of over 200 discrete documents that must be managed before, during, and after the trial. Thus, the high costs associated with electronic trial master file systems emerge as one of the major constraints for the growth of the market. With the increasing need to comply with stringent and proliferating regulatory requirements, ensure speedy clinical trials, reduce costs to maintain the electronic trial master file, require real-time audit-ready trial master file systems across all stages of clinical trials. However, vendors are developing electronic trial master systems that allow end-users to manage the complete lifecycle of content, from draft to the final approval, in a cost-effective manner.
This electronic trial master file systems market analysis report also provides detailed information on other upcoming trends and challenges that will have a far-reaching effect on the market growth. The actionable insights on the trends and challenges will help companies evaluate and develop growth strategies for 2021-2025.
Parent Market Analysis
Technavio categorizes the global electronic trial master file systems market as a part of the global information technology spending market. Our research report has extensively covered external factors influencing the parent market growth potential in the coming years, which will determine the levels of growth of the electronic trial master file systems market during the forecast period.
Who are the Major Electronic Trial Master File Systems Market Vendors?
The report analyzes the market’s competitive landscape and offers information on several market vendors, including:
- Aurea Corp.
- IQVIA Holdings Inc.
- Laboratory Corp. of America Holdings
- MasterControl Inc.
- Oracle Corp.
- Phlexglobal Ltd.
- SureClinical Inc.
- TransPerfect Global Inc.
- Veeva Systems Inc.
- WIRB Copernicus Group
This statistical study of the electronic trial master file systems market encompasses successful business strategies deployed by the key vendors. The electronic trial master file systems market is fragmented and the vendors are deploying organic and inorganic growth strategies to compete in the market.
Product Insights and News
-
Aurea Corp. - The company is involved in offering electronic trial master files that can track critical trial documents and global submissions.
To make the most of the opportunities and recover from post COVID-19 impact, market vendors should focus more on the growth prospects in the fast-growing segments, while maintaining their positions in the slow-growing segments.
The electronic trial master file systems market forecast report offers in-depth insights into key vendor profiles. The profiles include information on the production, sustainability, and prospects of the leading companies.
Electronic Trial Master File Systems Market Value Chain Analysis
Our report provides extensive information on the value chain analysis for the electronic trial master file systems market, which vendors can leverage to gain a competitive advantage during the forecast period. The end-to-end understanding of the value chains is essential in profit margin optimization and evaluation of business strategies. The data available in our value chain analysis segment can help vendors drive costs and enhance customer services during the forecast period.
The value chain of the information technology spending market includes the following core components:
- Research and development
- Developers or manufacturers
- Sales and distribution
- End-users
The report has further elucidated on other innovative approaches being followed by service providers to ensure a sustainable market presence.
Which are the Key Regions for Electronic Trial Master File Systems Market?
For more insights on the market share of various regions Request for a FREE sample now!
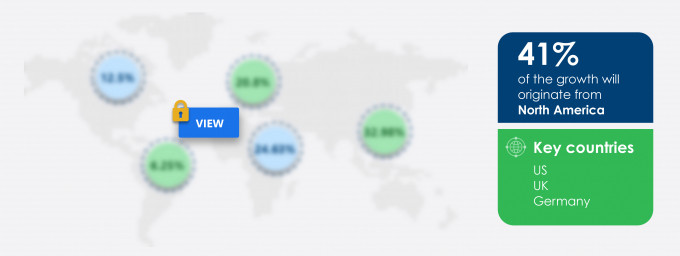
41% of the market’s growth will originate from North America during the forecast period. The US is the key market for electronic trial master file systems in North America. Market growth in this region will be faster than the growth of the market in the European, MEA, and South American regions.
The high number of pharmaceutical and biotechnology companies in the region, considerable investments in clinical trial research, and growing drug discoveries in the region will facilitate the electronic trial master file systems market growth in North America over the forecast period. This market research report entails detailed information on the competitive intelligence, marketing gaps, and regional opportunities in store for vendors, which will assist in creating efficient business plans.
COVID Impact and Recovery Analysis
Since the start of 2020, pharmaceutical companies in North America have been prioritizing research and development of COVID-19 related diagnostics, treatments, health technologies, and vaccines, as well as looking at the potential of existing treatments. Pharmaceutical companies have been collaborating with healthcare authorities and institutions on COVID-19-related clinical research, which has helped drive the growth of the electronic trial master file systems market in North America in 2020-2021. Thus, the growing clinical trials in the region will spur the demand for electronic trial master file systems and, in turn, drive market growth during the forecast period.
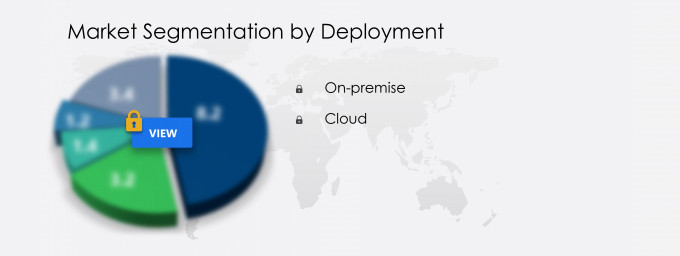
What are the Revenue-generating Deployment Segments in the Electronic Trial Master File Systems Market?
To gain further insights on the market contribution of various segments Request for a FREE sample
The electronic trial master file systems market share growth by the on-premise segment will be significant during the forecast period. The demand for electronic trial master file systems from pharmaceutical and biotechnology companies witnessed significant growth in 2020 for the effective planning, management, and tracking of their clinical study portfolios for COVID-19. Vendors operating in the market in focus entered into long-term partnerships with biotech and pharma companies to enhance clinical trial experiences.
This report provides an accurate prediction of the contribution of all the segments to the growth of the electronic trial master file systems market size and actionable market insights on post COVID-19 impact on each segment.
|
Electronic Trial Master File Systems Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
120 |
|
Base year |
2020 |
|
Forecast period |
2021-2025 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 11.65% |
|
Market growth 2021-2025 |
$ 679.48 million |
|
Market structure |
Fragmented |
|
YoY growth (%) |
10.83 |
|
Regional analysis |
North America, Europe, APAC, MEA, and South America |
|
Performing market contribution |
North America at 41% |
|
Key consumer countries |
US, UK, Germany, China, and Japan |
|
Competitive landscape |
Leading companies, Competitive strategies, Consumer engagement scope |
|
Key companies profiled |
Aurea Corp., IQVIA Holdings Inc., Laboratory Corp. of America Holdings, MasterControl Inc., Oracle Corp., Phlexglobal Ltd., SureClinical Inc., TransPerfect Global Inc., Veeva Systems Inc., and WIRB Copernicus Group |
|
Market dynamics |
Parent market analysis, Market growth inducers and obstacles, Fast-growing and slow-growing segment analysis, COVID 19 impact and recovery analysis and future consumer dynamics, Market condition analysis for the forecast period |
|
Customization purview |
If our report has not included the data that you are looking for, you can reach out to our analysts and get segments customized. |
What are the Key Data Covered in this Electronic Trial Master File Systems Market Report?
- CAGR of the market during the forecast period 2021-2025
- Detailed information on factors that will drive electronic trial master file systems market growth during the next five years
- Precise estimation of the electronic trial master file systems market size and its contribution to the parent market
- Accurate predictions on upcoming trends and changes in consumer behavior
- The growth of the electronic trial master file systems industry across North America, Europe, APAC, MEA, and South America
- A thorough analysis of the market’s competitive landscape and detailed information on vendors
- Comprehensive details of factors that will challenge the growth of electronic trial master file systems market vendors
We can help! Our analysts can customize this report to meet your requirements. Get in touch