Raloxifene Hydrochloride Market Size 2025-2029
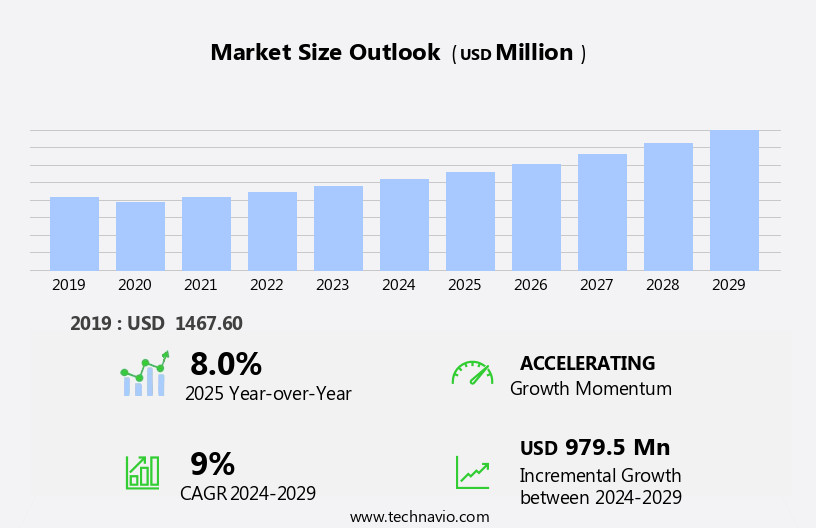
The raloxifene hydrochloride market size is forecast to increase by USD 979.5 million, at a CAGR of 9% between 2024 and 2029.
- The market is experiencing significant growth due to the increasing prevalence of osteoporosis and the expanding use of raloxifene HCl for breast cancer prevention. Osteoporosis, a condition characterized by weakened bones, affects millions worldwide, leading to an increased demand for effective treatments. Raloxifene HCl, a selective estrogen receptor modulator, is approved for the prevention and treatment of osteoporosis, making it a key player in this market. However, the market faces challenges related to the side effects of raloxifene HCl. Common side effects include hot flashes, leg cramps, and increased risk of thromboembolic events. These side effects can limit patient compliance and hinder market growth.
- Despite these challenges, opportunities exist for companies to innovate and develop new formulations or delivery methods to mitigate side effects and improve patient experience. By focusing on addressing these challenges and capitalizing on the growing demand for osteoporosis treatments, market participants can effectively navigate the competitive landscape and capitalize on the potential of the market.
What will be the Size of the Raloxifene Hydrochloride Market during the forecast period?
In the ever-evolving pharmaceutical landscape, the market for raloxifene hydrochloride continues to unfold, driven by its diverse applications across various sectors. This selective estrogen receptor modulator (SERM) is widely used in managing menopausal symptoms, improving bone mineral density, and reducing the risk of fractures in postmenopausal women. Raloxifene Hydrochloride's therapeutic index lies in its tissue selectivity, exhibiting estrogenic effects on bones and cardiovascular system while acting as an anti-estrogen in breast tissue. Its impact on estrogen receptor alpha and beta signaling pathways plays a crucial role in bone metabolism, bone resorption, and bone formation. The ongoing research in molecular biology and drug discovery is continually uncovering new insights into Raloxifene Hydrochloride's role in estrogen receptor-mediated cell signaling pathways and its potential applications in breast cancer prevention.
As healthcare providers and patient populations increasingly focus on personalized medicine, the drug's role in precision medicine and drug resistance is gaining significant attention. Market dynamics are influenced by various factors, including drug efficacy, drug interactions, drug elimination, regulatory approvals, pricing strategies, and patient education. The evolving patterns in these areas are shaping the market access and dosage forms of Raloxifene Hydrochloride, with a growing emphasis on patient satisfaction, health outcomes, and quality of life. The ongoing research in drug development is also addressing the challenges of drug tolerance and drug metabolism, leading to advancements in treatment protocols and healthcare policy.
As women at risk of osteoporosis and cardiovascular disease seek effective interventions, the demand for Raloxifene Hydrochloride is expected to remain robust. In the face of increasing competition from generic formulations and evolving clinical guidelines, companies are exploring new strategies to maintain their market position. These include improving drug safety, expanding indications, and enhancing patient education. The ongoing regulatory approvals and patent protection are crucial in ensuring the drug's continued efficacy and market access. Despite the challenges, the market remains a dynamic and promising space, with ongoing research in calcium homeostasis, estrogen replacement therapy, and endometrial cancer adding to its potential applications.
The future of this market lies in its ability to adapt to the evolving needs of patients and healthcare providers, with a focus on improving health outcomes and enhancing the quality of life for postmenopausal women.
How is this Raloxifene Hydrochloride Industry segmented?
The raloxifene hydrochloride industry research report provides comprehensive data (region-wise segment analysis), with forecasts and estimates in "USD million" for the period 2025-2029, as well as historical data from 2019-2023 for the following segments.
- End-user
- Pharmaceutical
- Chemical industry
- Type
- Tablet
- Branded vs. Generic
- Distribution Channel
- Hospital pharmacies
- Online pharmacies
- Pharmacy
- Retail pharmacies
- Application
- Postmenopausal Osteoporosis
- Breast Cancer Risk Reduction
- Others
- Geography
- North America
- US
- Canada
- Europe
- France
- Germany
- Italy
- UK
- APAC
- China
- India
- Japan
- South Korea
- Rest of World (ROW)
- North America
By End-user Insights
The pharmaceutical segment is estimated to witness significant growth during the forecast period.
The market for raloxifene Hydrochloride, a selective estrogen receptor modulator (SERM), is experiencing growth due to the rising prevalence of postmenopausal osteoporosis and the need for effective treatments. Raloxifene Hydrochloride, primarily marketed as Evista, is an antiresorptive drug that inhibits bone resorption and increases bone mineral density. It is also used for breast cancer prevention in postmenopausal women. The drug's selective binding to estrogen receptor alpha and beta, and tissue selectivity, make it an attractive therapeutic option. Clinical guidelines recommend raloxifene Hydrochloride as a first-line therapy for postmenopausal women with osteoporosis, as it reduces the risk of fractures.
Its efficacy is well-established through numerous clinical trials. The drug's elimination half-life allows for once-daily oral administration, making it a convenient treatment option. The safety profile of raloxifene Hydrochloride is a significant factor in its market success. It has a therapeutic index that makes it a preferred choice over estrogen replacement therapy for women at risk of cardiovascular disease or breast cancer. Patent protection for raloxifene Hydrocloride has expired, leading to the availability of generic formulations, which has influenced pricing strategies. Drug resistance and drug metabolism are ongoing areas of research to improve the drug's efficacy.
The drug's mechanism of action involves cell signaling pathways that regulate bone metabolism and estrogen receptor signaling. Healthcare providers prescribe raloxifene Hydrochloride to women with osteoporosis to improve bone health and reduce fracture risk. Patient education and treatment protocols are crucial to ensure optimal drug tolerance and patient satisfaction. The drug's impact on healthcare policy and market access is significant, as it addresses a critical healthcare need for women with osteoporosis. Adverse effects, such as hot flashes and night sweats, are manageable with proper dosage forms and patient education. The drug's potential applications in precision medicine and personalized treatment plans are areas of ongoing research.
In summary, the market is driven by the need for effective antiresorptive therapies for postmenopausal women with osteoporosis. Its selective binding, tissue selectivity, and safety profile make it a preferred choice over other treatments. Ongoing research in drug metabolism, drug resistance, and precision medicine will continue to influence the market's dynamics and trends.
The Pharmaceutical segment was valued at USD 996.60 million in 2019 and showed a gradual increase during the forecast period.
Regional Analysis
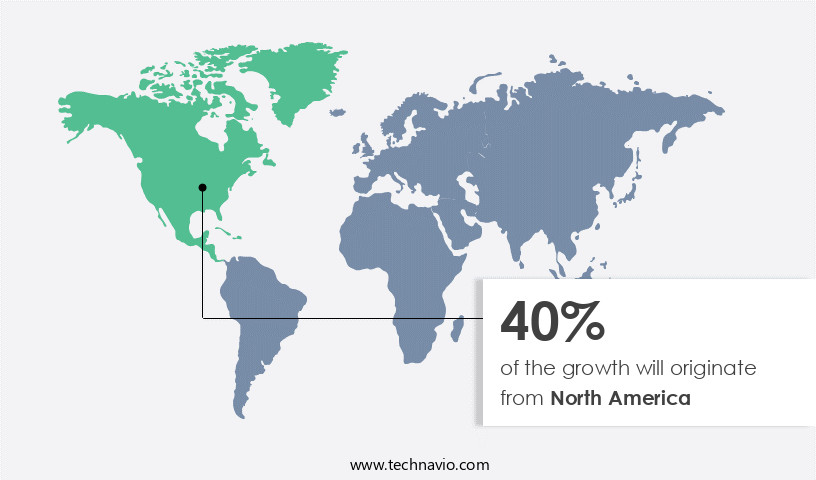
North America is estimated to contribute 40% to the growth of the global market during the forecast period.Technavio's analysts have elaborately explained the regional trends and drivers that shape the market during the forecast period.
The market in North America is experiencing significant growth due to the rising prevalence of postmenopausal osteoporosis and the availability of reimbursement schemes and health insurance coverage for related drugs. Major pharmaceutical companies, such as Amgen Inc., Eli Lilly and Company, and Pfizer Inc., are key players in this market. Raloxifene HCl's efficacy in reducing bone resorption and increasing bone mineral density makes it a popular choice for postmenopausal women at risk of fractures. The drug's selective binding to estrogen receptor alpha and tissue selectivity in breast tissue contribute to its therapeutic index, making it an alternative to estrogen replacement therapy for breast cancer prevention.
Regulatory approvals, including the U.S. Food and Drug Administration's approval for the prevention and treatment of osteoporosis, have further boosted the market. Patent protection and drug safety concerns have influenced pricing strategies, leading to the availability of generic formulations. However, drug resistance and adverse effects, such as hot flashes and night sweats, are challenges to patient satisfaction and market access. The ongoing research in molecular biology and cell signaling pathways aims to improve drug metabolism and resistance. Precision medicine and personalized treatment protocols based on patient populations and individual health outcomes are emerging trends. Healthcare providers and patients are increasingly focusing on quality of life and fracture risk reduction, leading to a growing demand for raloxifene HCl drugs.
The drug's cardiovascular benefits, including a reduced risk of cardiovascular disease, and its oral administration make it a preferred choice for many patients. The manufacturing process involves careful drug discovery, target identification, and receptor signaling to ensure optimal efficacy and safety. The therapeutic index, bone metabolism, and fracture risk reduction are crucial factors in the drug's success. In conclusion, the market in North America is driven by the increasing incidence and prevalence of postmenopausal osteoporosis, regulatory approvals, and reimbursement schemes. The drug's efficacy, safety, and patient satisfaction make it a preferred choice for healthcare providers and patients.
Ongoing research in molecular biology and precision medicine aims to address challenges such as drug resistance and adverse effects, ensuring the continued growth and success of the market.
Market Dynamics
Our researchers analyzed the data with 2024 as the base year, along with the key drivers, trends, and challenges. A holistic analysis of drivers will help companies refine their marketing strategies to gain a competitive advantage.
What are the key market drivers leading to the rise in the adoption of Raloxifene Hydrochloride Industry?
- The escalating incidence of osteoporosis serves as the primary catalyst fueling market growth.
- Osteoporosis, a condition characterized by bone resorption and decreased bone density, affects an estimated 200 million women worldwide, with a higher prevalence in older women. In the US and Europe, approximately 30% of postmenopausal women suffer from this condition, making it a significant public health concern. The aging population, driven by improved healthcare facilities and extended life expectancy, further increases the number of individuals at risk. Among ethnicities, Mexican American women have a higher risk, with one in four affected. Raloxifene hydrochloride, a selective estrogen receptor modulator (SERM), is a popular treatment for osteoporosis.
- It helps reduce fracture risk by inhibiting bone resorption and stimulating bone formation. However, drug resistance and varying responses to treatment due to cell signaling pathways necessitate precision medicine and individualized treatment protocols. Clinical trials play a crucial role in drug development, ensuring the safety and efficacy of Raloxifene hydrochloride. In the context of healthcare policy, patient education is essential to ensure proper usage and potential side effects, such as night sweats and hot flashes associated with hormone therapy, are well-understood. Drug metabolism and individual differences in metabolism further complicate treatment, necessitating ongoing research and development.
- In conclusion, the growing prevalence of osteoporosis, driven by an aging population, requires effective treatment options like Raloxifene hydrochloride. Precision medicine, clinical trials, and patient education are essential to optimize treatment outcomes and minimize side effects. Continuous research and development efforts are necessary to address drug resistance and individual differences in drug metabolism.
What are the market trends shaping the Raloxifene Hydrochloride Industry?
- The use of raloxifene HCl for the prevention of breast cancer is gaining significant traction in the market. This trend reflects growing recognition of its efficacy and potential health benefits.
- Raloxifene hydrochloride is a selective estrogen receptor modulator (SERM) that has gained significant attention for its role in managing menopausal symptoms and preventing breast cancer in postmenopausal women. The market growth for raloxifene hydrochloride is driven by its ability to improve bone mineral density and reduce the risk of invasive breast cancer. This preventive aspect is crucial as breast cancer remains a prevalent health concern worldwide. Raloxifene hydrochloride functions as a SERM, inhibiting estrogenic effects on breast tissue and stimulating bone formation. Its therapeutic index is well-established through extensive clinical trials and research studies.
- The medication's adverse effects are generally manageable, and its impact on endometrial cancer risk is minimal. The quality of life for postmenopausal women is significantly improved through the use of raloxifene hydrochloride, as it alleviates symptoms such as hot flashes and night sweats. Additionally, its dosage forms offer flexibility for patients, making it an attractive option for personalized medicine. The market access for raloxifene hydrochloride is facilitated by its proven efficacy in breast cancer prevention and its positive impact on bone metabolism. The therapeutic index of raloxifene hydrochloride is favorable, as it offers both preventive and therapeutic benefits.
- Overall, the growing awareness of breast cancer and the need for effective preventive measures are expected to propel the demand for raloxifene hydrochloride in the healthcare industry.
What challenges does the Raloxifene Hydrochloride Industry face during its growth?
- The challenge of managing side effects associated with osteoporosis medications is a significant factor impeding the growth of the industry.
- Raloxifene hydrochloride is a selective estrogen receptor modulator (SERM) used primarily for the prevention and treatment of postmenopausal osteoporosis. Its mechanism of action involves selective binding to estrogen receptors in certain tissues, leading to estrogenic effects in bones and anti-estrogenic effects in other tissues. However, drug interactions with other medications, such as warfarin and cyclosporine, can impact drug elimination and increase the risk of side effects. Clinical guidelines recommend raloxifene hydrochloride for women at risk of developing osteoporosis due to age, family history, or other risk factors. Drug safety concerns, including an increased risk of deep vein thrombosis and pulmonary embolism, especially in women with a history of cardiovascular disease, have led to regulatory approvals and patent protection for the drug.
- Despite its efficacy in treating osteoporosis, the market growth for raloxifene hydrochloride may be negatively impacted by the availability of generic formulations and pricing strategies. As a result, the market dynamics are complex, requiring a thorough understanding of drug interactions, clinical guidelines, and drug safety to effectively compete in this market.
Exclusive Customer Landscape
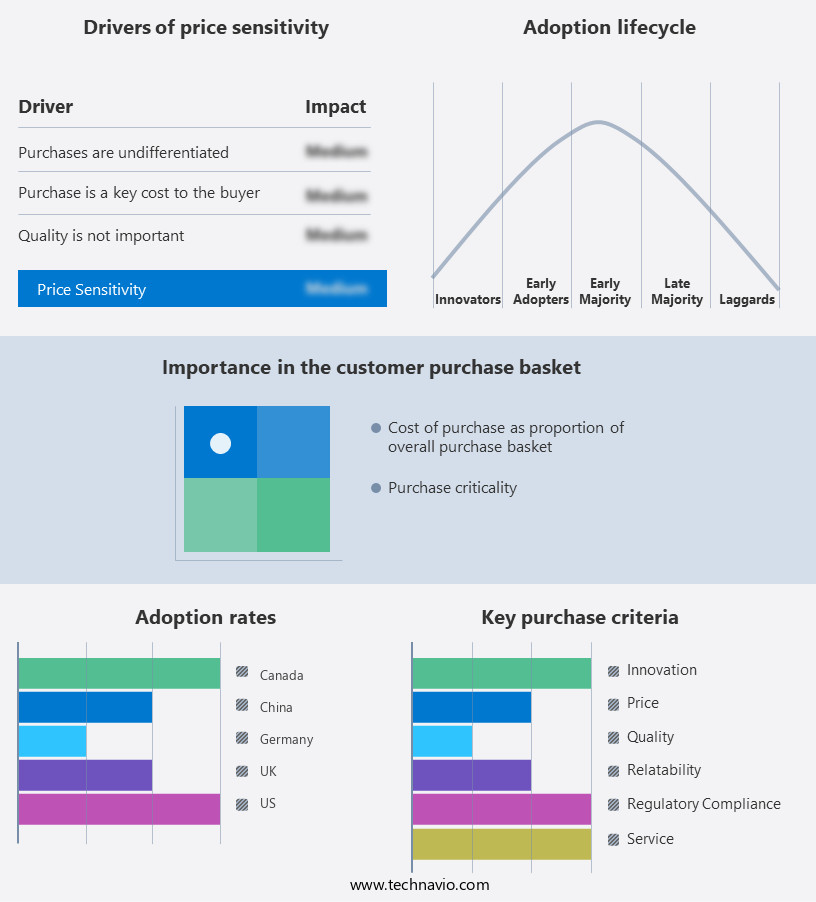
The raloxifene hydrochloride market forecasting report includes the adoption lifecycle of the market, covering from the innovator's stage to the laggard's stage. It focuses on adoption rates in different regions based on penetration. Furthermore, the raloxifene hydrochloride market report also includes key purchase criteria and drivers of price sensitivity to help companies evaluate and develop their market growth analysis strategies.
Customer Landscape
Key Companies & Market Insights
Companies are implementing various strategies, such as strategic alliances, raloxifene hydrochloride market forecast, partnerships, mergers and acquisitions, geographical expansion, and product/service launches, to enhance their presence in the industry.
Amneal Pharmaceuticals Inc. - Raloxifene hydrochloride, marketed as Raloxifene Amneal, is a crucial medication for postmenopausal women to prevent and manage osteoporosis. This selective estrogen receptor modulator (SERM) functions by binding to estrogen receptors, mimicking some of the protective effects of estrogen on bones while minimizing its unwanted side effects on other tissues. By inhibiting bone resorption and stimulating bone formation, Raloxifene Amneal plays a pivotal role in preserving bone health and reducing the risk of fractures. This medication is a valuable addition to the pharmacological arsenal for healthcare professionals addressing the health needs of their female patients in the menopausal stage.
The industry research and growth report includes detailed analyses of the competitive landscape of the market and information about key companies, including:
- Amneal Pharmaceuticals Inc.
- Blanver
- Cadila Pharmaceuticals Ltd.
- Cipla Inc.
- Dr Reddys Laboratories Ltd.
- Eli Lilly and Co.
- Enzo Biochem Inc.
- Glenmark Pharmaceuticals Ltd.
- Midas Pharma GmbH
- Santa Cruz Biotechnology Inc.
- Sigma Aldrich Chemicals Pvt. Ltd.
- Taj Pharmaceutical Ltd.
- Teva Pharmaceutical Industries Ltd.
- Thermo Fisher Scientific Inc.
- Tokyo Chemical Industry Co. Ltd.
Qualitative and quantitative analysis of companies has been conducted to help clients understand the wider business environment as well as the strengths and weaknesses of key industry players. Data is qualitatively analyzed to categorize companies as pure play, category-focused, industry-focused, and diversified; it is quantitatively analyzed to categorize companies as dominant, leading, strong, tentative, and weak.
Recent Development and News in Raloxifene Hydrochloride Market
- In March 2023, Eli Lilly and Company announced the approval of their Raloxifene Hydrochloride tablets by the European Commission for the prevention and treatment of osteoporosis in postmenopausal women at high risk of fracture (EMA, 2023). This expansion of the product's indication broadens its application and addresses a significant unmet medical need.
- In July 2024, Ipsen Pharma Biotech and Amgen Inc. Entered into a strategic partnership to co-promote Raloxifene Hydrochloride in Europe and other select countries (Ipsen, 2024). This collaboration strengthens both companies' positions in the osteoporosis market and enhances their ability to reach more patients.
- In October 2024, the U.S. Food and Drug Administration (FDA) granted priority review to a new formulation of Raloxifene Hydrochloride from Sun Pharmaceutical Industries Ltd. For the prevention and treatment of osteoporosis in postmenopausal women (Sun Pharma, 2024). The new formulation aims to improve patient compliance and convenience.
- In February 2025, Raloxifene Hydrochloride sales surpassed USD2 billion globally for the first time, driven by increasing demand and market penetration (Pharmaceutical Technology, 2025). This significant milestone underscores the market's potential and the product's importance in addressing osteoporosis.
- Sources:
- European Medicines Agency (EMA). (2023, March 17). European Commission approves new indication for Lilly's EVISTA (raloxifene) in the European Union. Retrieved from https://www.Ema.Europa.Eu/en/news/european-commission-approves-new-indication-lillys-evista-raloxifene-european-union
- Ipsen. (2024, July 13). Ipsen and Amgen Announce Strategic Collaboration to Co-promote Raloxifene in Europe and Select Other Countries. Retrieved from https://www.Ipsen.Com/en/news/press-releases/detail/ipsen-and-amgen-announce-strategic-collaboration-to-copromote-raloxifene-in-Europe-and-select-other-countries/
- Sun Pharmaceutical Industries Ltd. (2024, October 12). Sun Pharma's Raloxifene Hydrochloride Tablets USAN granted Priority Review by FDA. Retrieved from https://www.Sunpharma.Com/news-and-events/press-releases/press-release-details/2024/Sun-Pharmas-Raloxifene-Hydrochloride-Tablets-USAN-granted-Priority-Review-by-FDA/default.Aspx
- Pharmaceutical Technology. (2025, February 24). Raloxifene Hydrochloride sales exceed USD2 billion for the first time. Retrieved from https://www.Pharmaceutical-technology.Com/news/raloxifene-hydrochloride-sales-exceed-2-billion/
Research Analyst Overview
- In the biopharmaceutical industry, pharmaceutical marketing for estrogen deficiency treatments, particularly estrogen receptor modulators like raloxifene hydrochloride, continues to shape clinical practice guidelines. The menopausal transition, characterized by hormonal imbalance and bone loss, necessitates effective therapeutic options for fracture prevention. Raloxifene hydrochloride, approved for osteoporosis treatment, also offers cardiovascular risk reduction. Clinical trial design and patient management are crucial in ensuring drug safety monitoring and adverse event reporting. Healthcare innovation emphasizes personalized patient education materials and cost-benefit analysis. Therapeutic alternatives and treatment strategies are under constant evaluation in the context of health economics. Pharmaceutical regulation plays a significant role in drug promotion and drug monitoring.
- Estrogen receptor modulators, including raloxifene hydrochloride, face ongoing scrutiny for their clinical data analysis and off-label use. Clinical practice guidelines and therapeutic options are influenced by these regulatory decisions. The biopharmaceutical industry strives to address the challenges of hormonal imbalance and bone loss, fostering innovation and improving patient care. Effective patient management, drug safety monitoring, and cost-benefit analysis are essential components of osteoporosis treatment and overall healthcare innovation.
Dive into Technavio's robust research methodology, blending expert interviews, extensive data synthesis, and validated models for unparalleled Raloxifene Hydrochloride Market insights. See full methodology.
|
Market Scope |
|
|
Report Coverage |
Details |
|
Page number |
215 |
|
Base year |
2024 |
|
Historic period |
2019-2023 |
|
Forecast period |
2025-2029 |
|
Growth momentum & CAGR |
Accelerate at a CAGR of 9% |
|
Market growth 2025-2029 |
USD 979.5 million |
|
Market structure |
Fragmented |
|
YoY growth 2024-2025(%) |
8.0 |
|
Key countries |
US, Canada, Germany, UK, China, France, Japan, Italy, India, and South Korea |
|
Competitive landscape |
Leading Companies, Market Positioning of Companies, Competitive Strategies, and Industry Risks |
What are the Key Data Covered in this Raloxifene Hydrochloride Market Research and Growth Report?
- CAGR of the Raloxifene Hydrochloride industry during the forecast period
- Detailed information on factors that will drive the growth and forecasting between 2025 and 2029
- Precise estimation of the size of the market and its contribution of the industry in focus to the parent market
- Accurate predictions about upcoming growth and trends and changes in consumer behaviour
- Growth of the market across North America, Europe, Asia, and Rest of World (ROW)
- Thorough analysis of the market's competitive landscape and detailed information about companies
- Comprehensive analysis of factors that will challenge the raloxifene hydrochloride market growth of industry companies
We can help! Our analysts can customize this raloxifene hydrochloride market research report to meet your requirements.